EBILFUMIN 30 MG CAPSULAS DURAS EFG


Cómo usar EBILFUMIN 30 MG CAPSULAS DURAS EFG
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Ebilfumin 30 mg cápsulas duras EFG
oseltamivir
Lea todo el prospecto detenidamente antes de empezar a tomar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto:
- Qué es Ebilfumin y para qué se utiliza
- Qué necesita saber antes de empezar a tomar Ebilfumin
- Cómo tomar Ebilfumin
- Posibles efectos adversos
- Conservación de Ebilfumin
- Contenido del envase e información adicional
1. Qué es Ebilfumin y para qué se utiliza
- Ebilfumin se utiliza en adultos, adolescentes, niños y lactantes (incluidos los bebés recién nacidos a término) para el tratamiento de la gripe(influenza). Puede ser utilizado cuando tiene los síntomas de la gripe y se sabe que el virus de la gripe está presente en la población.
- Ebilfumin se puede recetar también a adultos, adolescentes, niños y lactantes mayores de 1 año de edad para prevenir la gripecaso por caso, por ejemplo, si usted ha estado en contacto con alguien que tiene gripe.
- Ebilfumin puede recetarse a adultos, adolescentes, niños y lactantes (incluidos los bebés recién nacidos a término) como tratamiento preventivoen circunstancias excepcionales, por ejemplo, si hay una epidemia global de gripe (una pandemiade gripe) y la vacuna estacional de la gripe no pueda dar una protección suficiente.
Ebilfumin contiene oseltamivir, el cual pertenece a un grupo de medicamentos denominados inhibidores de la neuraminidasa. Estos medicamentos previenen la propagación del virus de la gripe dentro del cuerpo. Ayudan a aliviar o a prevenir los síntomas de la infección por el virus de la gripe.
La gripe es una infección causada por un virus. Los signos de la gripe a menudo incluyen fiebre repentina (más de 37,8 ºC), tos, moqueo o congestión nasal, dolores de cabeza, dolores musculares y cansancio extremo. Estos síntomas también pueden ser causados por otras infecciones. Una verdadera infección gripal sólo ocurre durante los brotes anuales (epidémicos), cuando los virus de la gripe están diseminados en la población. Fuera de los periodos epidémicos, los síntomas seudogripales están generalmente ocasionados por otro tipo de enfermedad.
2. Qué necesita saber antes de empezar a tomar Ebilfumin
No tome Ebilfumin
- si es alérgicoal oseltamivir o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6).
Si a usted le ocurre esto, consulte con su médico. No tome Ebilfumin.
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a tomar Ebilfumin.
Antes de tomar Ebilfumin, asegúrese de que el médico sabe
- si es alérgico a otros medicamentos
- si padece alguna enfermedad del riñón. Si es así, puede que sea necesario ajustar su dosis.
- si padece alguna enfermedad graveque requiera hospitalización inmediata.
- si su sistema inmunitariono funciona adecuadamente.
- si padece enfermedad del corazóno enfermedad respiratoriacrónicas.
Durante el tratamiento con Ebilfumin, comente a un médico inmediatamente
- si nota cambios en su comportamiento o estado de ánimo (acontecimientos neuropsiquiátricos), especialmente si se dieran en niños y adolescentes. Éstos pueden ser signos de efectos adversos raros pero graves.
Ebilfumin no es una vacuna
Ebilfumin no es una vacuna: sirve para tratar la infección o prevenir la propagación del virus de la gripe. Una vacuna le proporciona anticuerpos frente al virus. Ebilfumin no cambia la efectividad de la vacuna de la gripe y su médico le podría recetar ambos.
Uso de Ebilfumin con otros medicamentos
Informe a su médico o farmacéutico si está tomando, ha tomado recientemente o podría tener que tomar cualquier otro medicamento. Esto incluye los medicamentos adquiridos sin receta. Los siguientes medicamentos son particularmente importantes:
- clorpropamida (usada para tratar la diabetes)
- metotrexato (usado para tratar, por ejemplo, la artritis reumatoide)
- fenilbutazona (empleada para tratar el dolor y las inflamaciones)
- probenecid (usada para tratar la gota)
Embarazo y lactancia
Debe informar a su médico si está embarazada, cree que podría estar embarazada o tiene intención de quedarse embarazada para que su médico pueda decidir si Ebilfumin es adecuado en su caso.
Se desconocen los efectos sobre los lactantes. Debe informar a su médico si está en periodo de lactancia para que pueda decidir si Ebilfumin es adecuado en su caso.
Consulte a su médico o farmacéutico antes de utilizar este medicamento.
Conducción y uso de máquinas
Ebilfumin no tiene efecto sobre su capacidad para conducir o utilizar máquinas.
Ebilfumin contiene sodio
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por cápsula; esto es, esencialmente “exento de sodio”.
3. Cómo tomar Ebilfumin
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico.
En caso de duda, consulte de nuevo a su médico o farmacéutico.
Tome Ebilfumin tan pronto como le sea posible, lo ideal dentro de los dos días tras haber empezado a tener los síntomas de la gripe.
Dosis recomendadas
Para el tratamiento de la gripe, tome dos dosis diarias. Generalmente es conveniente tomar una dosis por la mañana y otra por la noche. Es importante completar el tratamiento entero de 5 días, incluso si empieza a sentirse mejor rápidamente.
Para pacientes adultos con un sistema inmune debilitado, el tratamiento continuará durante 10 días.
Para la prevención de la gripe o tras haber estado en contacto con una persona infectada, tome una dosis diaria durante 10 días. Lo mejor es tomar esta dosis por las mañanas con el desayuno.
En situaciones especiales, como puede ser en casos de gripe muy extendida o pacientes con el sistema inmune debilitado, el tratamiento continuará hasta 6 ó 12 semanas.
La dosis recomendada depende del peso corporal del paciente.Debe utilizar la cantidad de cápsulas o suspensión oral que le haya prescrito su médico.
Adultos, y adolescentes de 13 años o mayores
Peso corporal | Tratamiento de la gripe: dosis durante 5 días | Prevención de la gripe: dosis durante 10 días |
40 kg o más | 75 mg dos veces al día | 75 mg una vez al día |
Se pueden obtener 75 mg con una cápsula de 30 mg más una cápsula de 45 mg
Niños de 1 a 12 años
Peso corporal | Tratamiento de la gripe: dosis durante 5 días | Prevención de la gripe: dosis durante 10 días |
10 a 15 kg | 30 mg dos veces al día | 30 mg una vez al día |
Más de 15 kg y hasta 23 kg | 45 mg dos veces al día | 45 mg una vez al día |
Más de 23 kg y hasta 40 kg | 60 mg dos veces al día | 60 mg una vez al día |
Más de 40 kg | 75 mg dos veces al día | 75 mg una vez al día |
Se pueden obtener 75 mg con una cápsula de 30 mg más una cápsula de 45 mg
Lactantes menores de 1 año (de 0 a 12 meses)
La administración de Ebilfumin a lactantes menores de 1 año de edad para prevenir la gripe durante una pandemia se debe realizar en base al criterio del médico después de considerar el beneficio potencial frente a cualquier riesgo potencial para el lactante.
Peso corporal | Tratamiento de la gripe: dosis durante 5 días | Prevención de la gripe: dosis durante 10 días |
De 3 kg a más de 10 kg | 3 mg por kg de peso, dos veces al día | 3 mg por kg de peso, una vez al día |
mg por kg = mg por cada kilogramo de peso corporal del niño. Por ejemplo:
Si un niño de 6 meses pesa 8 kg, la dosis es
8 kg x 3 mg por kg = 24 mg
Forma de administración
Trague las cápsulas enteras con agua. No rompa ni mastique las cápsulas.
Ebilfumin puede tomarse con o sin alimentos, aunque si se toma con comida se puede reducir la posibilidad de sentir o tener malestar (náuseas y vómitos).
Las personas que tengan dificultad para tragar las cápsulaspueden usar un medicamento líquido (suspensión oral). Si éste no se encuentra disponible en su farmacia, puede preparar una forma líquida de Ebilfumin a partir de las cápsulas. Ver las instrucciones en el apartadoPreparación deEbilfumin líquido en casa.
Si toma más Ebilfumin del que debe
Deje de tomar Ebilfumin y consulte de inmediato al médico o farmacéutico.
En la mayoría de los casos de sobredosis, no se comunicaron efectos adversos. Cuando se comunicaron efectos adversos, fueron similares a los que se dieron con dosis normales y que se incluyen en la sección 4.
Se han comunicado más frecuentemente casos de sobredosis con oseltamivir en niños que en adultos y adolescentes. Se debe tener precaución cuando se prepare Ebilfumin líquido para los niños y cuando se administren las cápsulas o Ebilfumin líquido a los niños.
Si interrumpe la toma de Ebilfumin
No tome una dosis doble para compensar las dosis olvidadas.
Si interrumpe el tratamiento con Ebilfumin
No se producen efectos adversos cuando deja de tomar Ebilfumin. Pero si deja de tomar Ebilfumin antes de lo que le dijo su médico, pueden reaparecer los síntomas de la gripe. Complete siempre el periodo de tratamiento que le haya recetado su médico.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Desde la comercialización de oseltamivir, raramente se han comunicado los siguientes efectos adversos graves:
- Reacciones anafilácticas y anafilactoides: reacciones alérgicas graves, con hinchazón de cara y piel, sarpullido con picor, tensión arterial baja y dificultad para respirar
- Trastornos hepáticos (hepatitis fulminante, trastorno de la función hepática e ictericia): piel y blanco de los ojos amarillentos, cambio del color de las heces, cambios en el comportamiento
- Edema angioneurótico: hinchazón grave repentina de la piel principalmente alrededor del área de la cabeza y cuello, incluyendo ojos y lengua, con dificultad para respirar.
- Síndrome de Stevens-Johnson y necrólisis epidérmica tóxica: reacción alérgica complicada con posible amenaza para la vida, grave inflamación de la parte externa y posiblemente interna de la piel, inicialmente con fiebre, dolor de garganta, y fatiga, sarpullido en la piel, con formación de ampollas, descamación, y grandes áreas de la piel peladas, posible dificultad respiratoria y tensión arterial baja
- Hemorragia gastrointestinal: hemorragia prolongada del intestino grueso o vómito de sangre
- Trastornos neuropsiquiátricos, según se describen abajo.
Si nota cualquiera de estos síntomas, consiga ayuda médica inmediatamente.
Los efectos adversos comunicados más frecuentemente (muy frecuentes y frecuentes) para Ebilfumin son sensación de malestar o malestar (náuseas, vómitos), dolor de estómago, malestar de estómago, dolor de cabeza y dolor. Estos efectos adversos por lo general ocurren tras la primera dosis del medicamento y generalmente suelen desaparecer a lo largo del tratamiento. La frecuencia con que aparecen estos efectos se reduce si el medicamento se toma con alimentos.
Efectos adversos raros pero graves: consiga ayuda médica inmediatamente
(Éstos pueden afectar hasta a 1 de cada 1.000 personas)
Durante el tratamiento con oseltamivir se han comunicado efectos adversos raros que incluyen
- Convulsiones y delirio, incluyendo alteración en los niveles de consciencia
- Confusión, comportamiento anormal
- Trastornos delirantes, alucinaciones, agitación, ansiedad, pesadillas
Estos acontecimientos se comunicaron principalmente en niños y adolescentes y a menudo comenzaron de forma repentina y tuvieron una resolución rápida. En muy raras ocasiones estos acontecimientos tuvieron como resultado autolesión, algunos con desenlace mortal. Estos acontecimientos neuropsiquiátricos también se han comunicado en pacientes con gripe que no estaban tomando oseltamivir.
- Los pacientes, especialmente niños y adolescentes, deben ser estrechamente observados para detectar los cambios en el comportamiento descritos anteriormente.
Si nota cualquiera de estos síntomas, especialmente en los pacientes más jóvenes, consiga ayuda médica inmediatamente.
Adultos y adolescentes de 13 años en adelante
Efectos adversos muy frecuentes
(pueden afectar a más de 1 de cada 10 personas)
- Dolor de cabeza
- Náuseas
Efectos adversos frecuentes
(pueden afectar hasta a 1 de cada 10 personas)
- Bronquitis
- Calenturas
- Tos
- Mareo
- Fiebre
- Dolor
- Dolor en las extremidades
- Moqueo
- Dificultad para dormir
- Dolor de garganta
- Dolor de estómago
- Cansancio
- Sensación de plenitud en la parte superior del abdomen
- Infecciones de las vías respiratorias altas (inflamación de nariz, garganta y senos)
- Malestar de estómago
- Vómitos
Efectos adversos poco frecuentes
(pueden afectar hasta a 1 de cada 100 personas)
- Reacciones alérgicas
- Nivel de consciencia alterado
- Convulsión
- Alteraciones del ritmo del corazón
- Alteraciones de la función del hígado de leves a graves
- Reacciones en la piel (inflamación de la piel, sarpullido enrojecido y con picor, piel escamosa)
Efectos adversos raros
(pueden afectar hasta a 1 de cada 1.000 personas)
- Trombocitopenia (número reducido de plaquetas)
- Trastornos de la vista
Niños de 1 a 12 años
Efectos adversos muy frecuentes:
(pueden afectar a 1 de cada 10 personas)
- Tos
- Congestión nasal
- Vómitos
Efectos adversos frecuentes:
(pueden afectar hasta a 1 de cada 10 personas)
- Conjuntivitis (ojos enrojecidos y llorosos o dolor en los ojos)
- Inflamación de los oídos y otros trastornos en los oídos
- Dolor de cabeza
- Náuseas
- Moqueo
- Dolor de estómago
- Sensación de plenitud en la parte superior del abdomen
- Molestia de estómago
Efectos adversos poco frecuentes:
(pueden afectar hasta a 1 de cada 100 personas)
- Inflamación de la piel
- Trastorno de la membrana timpánica (tímpano)
Lactantes menores de 1 año
Los efectos adversos observados en lactantes de 0 a 12 meses de edad, son en su mayoría similares a los efectos adversos comunicados en niños mayores (a partir de 1 año). Además, se han comunicado diarrea y dermatitis del pañal.
Si considera que alguno de los efectos adversos que sufre es grave o si aprecia cualquier efecto adverso no mencionado en este prospecto, informe a su médico o farmacéutico. Sin embargo,
- si usted o su hijo están enfermos varias veces, o
- si los síntomas de la gripe empeoran o la fiebre continúa informe a su médico lo antes posible.
Comunicación de efectos adversos
Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Anexo V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Ebilfumin
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la caja y el blíster después de EXP. La fecha de caducidad es el último día del mes que se indica.
No conservar a temperatura superior a 25 °C.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Ebilfumin
- Cada cápsula dura contiene fosfato de oseltamivir equivalente a 30 mg de oseltamivir.
- Los demás componentes son:
contenido de la cápsula: almidón pregelatinizado (derivado del almidón de maíz), talco, povidona (K-29/32), croscarmelosa sódica y estearilfumarato sódico
cubierta de la cápsula: gelatina, óxido de hierro amarillo (E172) y dióxido de titanio (E171)
tinta de impresión: goma laca al 45 % (20 % esterificada), óxido de hierro negro (E172), propilenglicol (E1520), hidróxido de amonio al 28 % (E527)
Aspecto del producto y contenido del envase
La cápsula dura está formada por un cuerpo y una tapa de color amarillo fuerte, con la marca de impresión negra “OS 30”. Tamaño de la cápsula: 4.
Ebilfumin 30 mg cápsulas duras EFG está disponible en blísteres de 10 cápsulas.
Titular de la Autorización de Comercialización
Actavis Group PTC ehf.
Reykjavíkurvegi 76-78
220 Hafnarfjördur
Islandia
Responsable de la fabricación
Balkanpharma-Dupnitsa AD
3 Samokovsko Shosse Str.
Dupnitsa 2600
Bulgaria
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique/Belgien Actavis Group PTC ehf. IJsland/Islande/Island Tél/Tel: +354 5503300 | Lietuva UAB Teva Baltics Tel: +370 52660203 |
???????? ??????? ??? Te?: +359 24899585 | Luxembourg/Luxemburg Actavis Group PTC ehf. Islande/Island Tél/Tel: +354 5503300 |
Ceskárepublika Teva Pharmaceuticals CR, s.r.o. Tel: +420 251007111 | Magyarország Teva Gyógyszergyár Zrt. Tel: +36 12886400 |
Danmark Teva Denmark A/S Tlf: +45 44985511 | Malta Actavis Ltd. Tel: +356 21693533 |
Deutschland TEVA GmbH Tel: +49 73140208 | Nederland Teva Nederland B.V. Tel: +31 8000228400 |
Eesti UAB Teva Baltics Tel: +372 6610801 | Norge Teva Norway AS Tlf: +47 66775590 |
Ελλ?δα Specifar A.B.E.E Τηλ: +30 2118805000. | Österreich ratiopharm Arzneimittel Vertriebs-GmbH Tel: +43 1970070 |
España Teva Pharma, S.L.U. Tel: +34 913873280 | Polska Teva Pharmaceuticals Polska Sp. z o.o. Tel: +48 223459300 |
France Teva Santé Tél: +33 155917800 | Portugal Actavis Group PTC ehf. Islândia Tel: +354 5503300 |
Hrvatska Pliva Hrvatska d.o.o. Tel: +385 13720000 | România Teva Pharmaceuticals S.R.L. Tel: +40 212306524 |
Ireland Teva Pharmaceuticals Ireland Tel: +353 19127700 | Slovenija Pliva Ljubljana d.o.o. Tel: +386 15890390 |
Ísland Actavis Group PTC ehf. Sími: +354 5503300 | Slovenská republika TEVA Pharmaceuticals Slovakia s.r.o. Tel: +421 257267911 |
Italia Actavis Group PTC ehf. Islanda Tel: +354 5503300 | Suomi/Finland Teva Finland Oy Puh/Tel: +358 201805900 |
Κ?προς Specifar A.B.E.E. Τηλ: +30 2118805000 Ελλ?δα | Sverige Teva Sweden AB Tel: +46 42121100 |
Latvija UAB Teva Baltics filiale Latvija Tel: +371 67323666 | United Kingdom Teva UK Limited Tel: +44 1977628500 |
Fecha de la última revisión de este prospecto: {MM/AAAA} {mes AAAA}.
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos http://www.ema.europa.eu.
--------------------------------------------------------------------------------------------------------------------------
Información para el usuario
Para las personas que encuentren difícil tragar las cápsulas, incluyendo a niños muy pequeños, puede estar disponible un medicamento en forma líquida.
Si usted necesita un medicamento líquido, pero no está disponible, se puede preparar una suspensión en la farmacia a partir de Ebilfumin cápsulas (ver Información para profesionales del sectorsanitario).La preparación elaborada en la farmacia es la opción recomendada.
Si la preparación de la farmacia tampoco está disponible, usted puede preparar en casa una suspensión de Ebilfumin a partir de estas cápsulas.
La dosis es la misma para el tratamiento que para la prevención de la gripe. La diferencia es la frecuencia con la que se administra.
Preparación de Ebilfumin líquido en casa
- Si tiene la cápsula correctapara la dosis necesaria (una dosis de 30 mg o de 60 mg), abra la cápsula y agite su contenido en una cucharadita (o menos) de alimento edulcorado idóneo. Esto normalmente es adecuado para niños mayores de 1 año. Vea el apartado superior de lasinstrucciones.
- Si necesita dosis más pequeñas,la preparación de la suspensión de Ebilfumin a partir de las cápsulas requiere más pasos. Esto es adecuado para niños pequeños y bebés: generalmente necesitan una dosis de Ebilfumin de menos de 30 mg. Vea el apartado inferior de lasinstrucciones.
Niños de 1 hasta 12 años
Para hacer una dosis de 30 mg o 60 mg, necesitará:
- Una o dos cápsula(s) de Ebilfumin de 30 mg
- Unas tijeras afiladas
- Un recipiente pequeño
- Cucharilla (cucharilla de 5 ml)
- Agua
- Alimento edulcoradopara enmascarar el sabor amargo del polvo.
Ejemplos: sirope de chocolate o de cereza y salsas de postre, como caramelo o leche condensada. O puede preparar agua azucarada mezclando una cucharilla de agua con tres cuartos (3/4) de una cucharilla de azúcar.
Paso 1: Comprobar la dosis correcta
Para saber la cantidad correcta que hay que utilizar, busque el peso del paciente en la izquierda de la tabla.
Mire la columna de la derecha para ver el número de cápsulas que tendrá que dar al paciente para una dosis única. La cantidad es la misma para el tratamiento que para la prevención de la gripe.
Sólo debe usar las cápsulas de 30 mg para dosis de 30 y 60 mg. No intente preparar dosis de 45 mg o 75 mg utilizando el contenido de las cápsulas de 30 mg. En su lugar utilice la cápsula con la dosis apropiada.
Peso | Dosis de Ebilfumin | Número de cápsulas |
Hasta 15 kg | 30 mg | 1 cápsula |
15 kg hasta 23 kg | 45 mg | No utilizar cápsulas de 30 mg |
23 kg hasta 40 kg | 60 mg | 2 cápsulas |
Paso 2: Verter todo el polvo dentro del recipiente
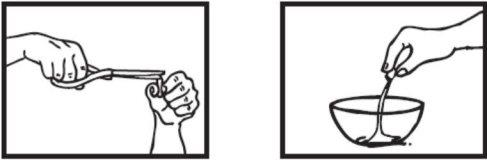
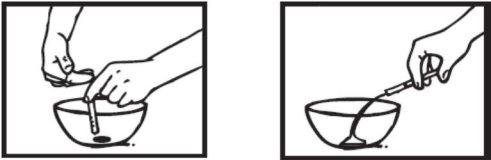
Coja una cápsula de 30 mgen posición vertical sobre un recipiente y recorte cuidadosamente con las tijeras el extremo redondeado.
Vierta todo el polvo dentro del recipiente.
Para una dosis de 60 mg abra una segunda cápsula. Vierta todo el polvo dentro del recipiente.
Tenga cuidado con el polvo, porque puede resultar irritante para la piel y ojos.
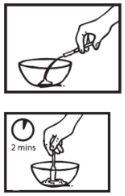
Paso 3: Endulzar el polvo y dar la dosis
Añada una cantidad pequeña -no más de una cucharilla- de alimento edulcorado al polvo que está en el recipiente.
Esto es para enmascarar el sabor amargo del polvo de Ebilfumin.
Agite bien la mezcla.
Dele inmediatamente al paciente todo el contenidodel recipiente.
Si queda algo de mezclaen el recipiente, enjuague el recipiente con una pequeña cantidad de agua y déselo al paciente para que tome todo.
Repita este procedimiento cada vez que necesite dar el medicamento.
Lactantes menores de 1 año
Para hacer una dosis única más pequeña, necesitará:
- Una cápsula de Ebilfumin 30 mg
- Unas tijeras afiladas
- Dos recipientes pequeños(utilizar pares de recipientes diferentes para cada niño)
- Un dispensador oral de dosis grandepara medir el agua-un dispensador de 5 ó 10 ml
- Un dispensador oral de dosis pequeñoque muestre medidas de 0,1 ml para dar la dosis
- Cucharilla (cucharilla de 5 ml)
- Agua
- Alimento edulcoradopara enmascarar el sabor amargo de Ebilfumin.
Ejemplos: sirope de chocolate o sirope de cereza y salsa de postre, como caramelo o leche condensada.
O puede preparar agua azucarada: mezclar una cucharilla de agua con tres cuartos (3/4) de una cucharilla de azúcar.
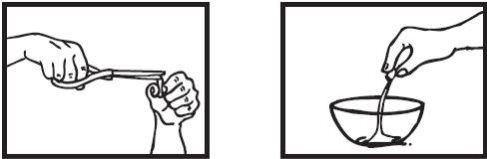
Paso 1: Verter todo el polvo dentro en un recipiente
Coja una cápsula de 30 mgen posición vertical sobre uno de los recipientes y recorte cuidadosamente con las tijeras el extremo redondeado. Tenga cuidado con el polvo: puede ser irritante para la piel y los ojos.
Vierta todo el polvo dentro del recipiente, independientemente de la dosis que esté preparando.
La cantidad es la misma para el tratamiento que para la prevención de la gripe.
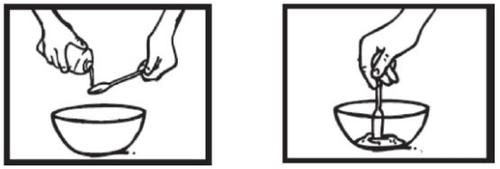
Paso 2: Añada agua para diluir el medicamento
Utilizar el dispensador más grande para extraer 5 mlde agua. Añada el agua al polvo que está en el recipiente. Agite la suspensión con una cucharilla durante 2 minutos. |
|
No se preocupe si no se disuelve todo el polvo. El polvo no disuelto se corresponde con ingredientes inactivos.
Paso 3: Elegir la cantidad correcta para el peso del niño
Busque en la columna de la izquierda de la tabla el peso del niño.
La columna derecha de la tabla muestra cuánta cantidad de la mezcla líquida necesitará preparar.
Lactantes menores de 1 año (incluidos los bebés recién nacidos a término)
Peso del niño (más próximo) | Cantidad de mezcla que hay que preparar |
3 kg | 1,5 ml |
3,5 kg | 1,8 ml |
4 kg | 2,0 ml |
4,5 kg | 2,3 ml |
5 kg | 2,5 ml |
5,5 kg | 2,8 ml |
6 kg | 3,0 ml |
6,5 kg | 3,3 ml |
7 kg | 3,5 ml |
7,5 kg | 3,8 ml |
8 kg | 4,0 ml |
8,5 kg | 4,3 ml |
9 kg | 4,5 ml |
9,5 kg | 4,8 ml |
10 kg o más | 5,0 ml |
Paso 4: Preparar la mezcla líquida
Asegúrese de que tiene el tamaño de dispensador correcto.
Elabore la cantidad correcta de mezcla líquida del primer recipiente.
Prepárela cuidadosamente para no coger burbujas de aire.
Añada suavemente la dosis correcta al segundo recipiente.
Paso 5: Endulzar y dársela al niño
Añada una cantidad pequeña –no más de una cucharilla- de alimento edulcorado al segundo recipiente.
Esto es para enmascarar el sabor amargo de la suspensión de Ebilfumin.
Agite bien el alimento edulcorado con el líquido de Ebilfumin.
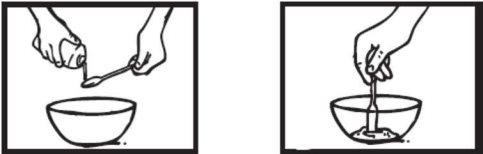
Dele inmediatamente al niño todo el contenidodel segundo recipiente (alimento edulcorado con la suspensión de Ebilfumin).
Si queda algo en el segundo recipiente, enjuague el recipiente con una pequeña cantidad de agua y consiga que el niño lo beba todo. Para niños que no puedan beber directamente del recipiente, déselo con una cuchara o emplee una botella adecuada para darle al niño el líquido que quedó.
Dele al niño algo para beber.
Deseche cualquier resto de líquido que haya sobrado del primer recipiente.
Repita este procedimiento cada vez que necesite dar el medicamento.
-------------------------------------------------------------------------------------------------------------------
Información únicamente para profesionales del sector sanitario:
Pacientes que no puedan tragar las cápsulas:
La presentación comercializada de oseltamivir polvo para suspensión oral (6 mg/ml) es el medicamento de elección para pacientes pediátricos y adultos que tengan dificultad para tragar las cápsulas o que necesiten dosis más bajas. En el caso de que oseltamivir polvo para suspensión oral no esté disponible, el farmacéutico puede preparar una suspensión (6 mg/ml) a partir de cápsulas de Ebilfumin. Si la suspensión preparada en la farmacia tampoco está disponible, los pacientes pueden preparar la suspensión en casa a partir de las cápsulas.
Se deben proporcionar los dispensadores orales de dosis(jeringas orales) con el volumen y graduación adecuados para administrar la suspensión preparada en la farmacia y para los procedimientos a seguir para la preparación en casa. En ambos casos, los volúmenes que se necesitan deben estar preferiblemente marcados en los dispensadores. Para la preparación en casa, se deben proporcionar aparte los dispensadores para coger el volumen correcto de agua y para medir la mezcla de Ebilfumin con agua. Para medir 5,0 ml de agua se deben utilizar dispensadores de 5 ml o 10 ml.
A continuación, se muestra el tamaño de dispensador adecuado para extraer el volumen correcto de la suspensión de Ebilfumin (6 mg/ml).
Lactantes menores de 1 año (incluidos los bebés recién nacidos a término):
Dosis de Ebilfumin | Cantidad de suspensión de Ebilfumin | Tamaño de dispensador a utilizar (graduado en 0,1 ml) |
9 mg | 1,5 ml | 2,0 ml (o 3,0 ml) |
10 mg | 1,7 ml | 2,0 ml (o 3,0 ml) |
11,25 mg | 1,9 ml | 2,0 ml (o 3,0 ml) |
12,5 mg | 2,1 ml | 3,0 ml |
13,75 mg | 2,3 ml | 3,0 ml |
15 mg | 2,5 ml | 3,0 ml |
16,25 mg | 2,7 ml | 3,0 ml |
18 mg | 3,0 ml | 3,0 ml (o 5,0 ml) |
19,5 mg | 3,3 ml | 5,0 ml |
21 mg | 3,5 ml | 5,0 ml |
22,5 mg | 3,8 ml | 5,0 ml |
24 mg | 4,0 ml | 5,0 ml |
25,5 mg | 4,3 ml | 5,0 ml |
27 mg | 4,5 ml | 5,0 ml |
28,5 mg | 4,8 ml | 5,0 ml |
30 mg | 5,0 ml | 5,0 ml |
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a EBILFUMIN 30 MG CAPSULAS DURAS EFGForma farmacéutica: CAPSULA, 75 mgPrincipio activo: oseltamivirFabricante: Actavis Group Ptc Ehf.Requiere recetaForma farmacéutica: CAPSULA, 30 mgPrincipio activo: oseltamivirFabricante: Accord Healthcare S.L.U.Requiere recetaForma farmacéutica: CAPSULA, 45 mgPrincipio activo: oseltamivirFabricante: Accord Healthcare S.L.U.Requiere receta
Médicos online para EBILFUMIN 30 MG CAPSULAS DURAS EFG
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de EBILFUMIN 30 MG CAPSULAS DURAS EFG, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes