
DURFENTA 100 micrograms/HOUR TRANSDERMAL PATCHES


How to use DURFENTA 100 micrograms/HOUR TRANSDERMAL PATCHES
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Durfenta 12 micrograms/hour transdermal patch EFG
Durfenta 25 micrograms/hour transdermal patch EFG
Durfenta 50 micrograms/hour transdermal patch EFG
Durfenta 75 micrograms/hour transdermal patch EFG
Durfenta 100 micrograms/hour transdermal patch EFG
fentanyl
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Durfenta and what is it used for
- What you need to know before you use Durfenta
- How to use Durfenta
- Possible side effects
- Storing Durfenta
- Contents of the pack and other information
1. What is Durfenta and what is it used for
This medicine is called Durfenta
The patches help to relieve very strong and long-lasting pain:
- in adults who need continuous pain treatment
- in children over 2 years of age who are already using opioid medication and who need continuous pain treatment.
Durfenta contains a medicine called fentanyl. It belongs to a group of strong pain-relieving medicines called opioids.
2. What you need to know before you use Durfenta
Do not use Durfenta:
- If you are allergic to fentanyl or any of the other ingredients of this medicine (listed in section 6).
- If you have short-term pain, such as sudden pain or pain after an operation.
- If you have breathing difficulties, with slow or weak breathing.
Do not use this medicine if you or your child are in any of the above situations. If you are not sure, consult your doctor or pharmacist before using Durfenta.
Warnings and precautions
|
If the patch sticks to another person
The patch should only be used on the skin of the person for whom the doctor has prescribed it. There have been cases where the patch has accidentally stuck to another family member through close physical contact or sharing the same bed with the person wearing the patch. If the patch accidentally sticks to another person (especially a child), the medicine in the patch can pass through the skin of the other person and can cause serious side effects such as breathing difficulties, with slow or weak breathing, which can be life-threatening. If the patch sticks to someone else's skin, it should be removed immediately and a doctor should be consulted.
Be careful with Durfenta
Consult your doctor or pharmacist beforeusing this medicine if you are in any of the following situations. Your doctor will need to monitor you more closely:
- If you have ever had lung or breathing problems.
- If you have ever had heart, liver or kidney problems, or low blood pressure.
- If you have ever had a brain tumor.
- If you have ever had persistent headaches or a head injury.
- If you are an elderly person, as you may be more sensitive to the effects of this medicine.
- If you have a condition called "myasthenia gravis", in which the muscles become weak and tired easily.
If you meet any of the above conditions (or if you are not sure), consult your doctor or pharmacist before using Durfenta.
While using the patch, tell your doctor if you have breathing problems while you are asleep.Opioids like Durfenta can cause sleep-related breathing disorders such as sleep apnea (breathing pauses while asleep) and sleep-related hypoxemia (low oxygen levels in the blood). Talk to your doctor if you, your partner or caregiver notice any of the following symptoms:
- breathing pauses while asleep
- waking up at night due to lack of breath
- difficulty staying asleep
- excessive sleepiness during the day.
Your doctor may decide to change the dose.
While using the patch, tell your doctor if you notice a change in the pain you feel.If you feel:
- that the patch no longer relieves the pain
- an increase in pain
- a change in the way you feel the pain (for example, you feel pain in another part of your body)
- pain when there should be no pain.
Do not change the dose yourself. Your doctor may decide to change the dose or change the treatment.
Side effects and Durfenta
- Durfenta can make you feel extremely sleepy and can slow down your breathing. In rare cases, these breathing problems can be life-threatening or even fatal, especially in people who have not used strong opioid painkillers before (such as Durfenta or morphine). If you, or your partner or caregiver, notice that the person wearing the patch is unusually sleepy, with slow or weak breathing:
- Remove the patch
- Call a doctor or go to the nearest hospital immediately
- Get the person to move and talk as much as possible
- If you have a fever while using Durfenta, talk to your doctor. This can increase the amount of medicine that passes through your skin.
- Durfenta can cause constipation; talk to your doctor or pharmacist for advice on how to prevent or relieve constipation.
In section 4, you can find the complete list of possible side effects.
When wearing the patch, do not expose it to direct heat, such as heat pads, electric blankets, hot water bottles, water beds, heat lamps or sunlamps. Do not take sunbaths or long hot baths and do not use saunas or hot tubs. If you do, you may increase the amount of medicine released from the patch.
Long-term use and tolerance
This medicine contains fentanyl, an opioid. Repeated use of opioid painkillers can make the medicine less effective (the body gets used to it, which is known as pharmacological tolerance). You may also become more sensitive to pain when using Durfenta. This is known as hyperalgesia. Increasing the dose of the patches may continue to reduce the pain for a while, but it can also be harmful. If you notice that the medicine is becoming less effective, consult your doctor. Your doctor will decide whether to increase the dose or gradually reduce the use of Durfenta.
Dependence and addiction
This medicine contains fentanyl, which is an opioid. It can cause dependence and/or addiction. |
Repeated use of Durfenta can also lead to dependence, abuse and addiction, which can result in a potentially life-threatening overdose. The risk of these side effects may be greater with a higher dose and longer use. Dependence or addiction can lead to a feeling of lack of control over the amount of medicine you need to use or how often you need to use it. You may feel the need to continue using the medicine even if it no longer helps to relieve the pain.
The risk of dependence or addiction varies from person to person. The risk of becoming dependent on or addicted to Durfenta may be greater if:
- You or any member of your family have abused alcohol or have been dependent on it, or have taken prescription medicines or illegal drugs ("addiction").
- You smoke.
- You have ever had mood problems (depression, anxiety or personality disorder) or have received treatment from a psychiatrist for other mental health problems.
If you notice any of the following symptoms while using Durfenta, it could be a sign of dependence or addiction.
- You need to use the medicine for longer than prescribed by your doctor.
- You need to use a higher dose than recommended.
- You are using the medicine for reasons other than those prescribed, for example, "to calm down" or "to help you sleep".
- You have made repeated unsuccessful attempts to stop using the medicine or control your use of it.
- You feel unwell when you stop using the medicine, and you feel better once you start taking it again ("withdrawal symptoms").
If you notice any of these signs, consult your doctor to determine the best course of treatment for you, when it is appropriate to stop the medicine, and how to do it safely.
Withdrawal symptoms when stopping Durfenta
Do not stop using this medicine suddenly. Withdrawal symptoms such as restlessness, difficulty sleeping, irritability, agitation, anxiety, feeling your heartbeat (palpitations), increased blood pressure, feeling sick, diarrhea, loss of appetite, tremors, chills or sweating may occur. If you want to stop using this medicine, talk to your doctor first. Your doctor will tell you how to do it, usually by gradually reducing the dose so that the unpleasant withdrawal effects are minimized.
Other medicines and Durfenta
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines. This includes medicines that you buy without a prescription and herbal medicines. You should also tell your pharmacist that you are using Durfenta if you buy any medicines from the pharmacy.
Your doctor will know which medicines can be taken safely with Durfenta. You may need to be monitored closely if you are taking any of the following types of medicines, or if you stop taking any of the following types of medicines, as this can affect the dose of Durfenta that you need.
In particular, tell your doctor or pharmacist if you are taking:
- Other pain medicines, such as other strong opioid painkillers (e.g. buprenorphine, nalbuphine or pentazocine) and some painkillers for nerve pain (gabapentin and pregabalin).
- Medicines to help you sleep (such as temazepam, zaleplon or zolpidem).
- Medicines to help you calm down (tranquilizers, such as alprazolam, clonazepam, diazepam, hydroxyzine or lorazepam) and medicines for mental health problems (antipsychotics, such as aripiprazole, haloperidol, olanzapine, risperidone or phenothiazines).
- Medicines to relax your muscles (such as cyclobenzaprine or diazepam).
- Some medicines used to treat depression called SSRIs or SNRIs (such as citalopram, duloxetine, escitalopram, fluoxetine, fluvoxamine, paroxetine, sertraline, venlafaxine). – see below for more information.
- Some medicines used to treat depression or Parkinson's disease called MAOIs (such as isocarboxazid, phenelzine, selegiline or tranylcypromine). You should not use Durfenta in the 14 days after stopping these medicines. – see below for more information.
- Some antihistamines, especially those that cause drowsiness (such as chlorpheniramine, clemastine, cyproheptadine, diphenhydramine or hydroxyzine).
- Some antibiotics used to treat infections (such as erythromycin or clarithromycin).
- Medicines used to treat fungal infections (such as itraconazole, ketoconazole, fluconazole or voriconazole).
- Medicines used to treat HIV infection (such as ritonavir).
- Medicines used to treat irregular heartbeats (such as amiodarone, diltiazem or verapamil).
- Medicines used to treat tuberculosis (such as rifampicin).
- Some medicines used to treat epilepsy (such as carbamazepine, phenobarbital or phenytoin).
- Some medicines used to treat nausea or vomiting (such as phenothiazines).
- Some medicines used to treat heartburn or stomach ulcers (such as cimetidine).
- Some medicines used to treat angina (chest pain) or high blood pressure (such as nicardipine).
- Some medicines used to treat blood cancer (such as idelalisib).
Using Durfenta with antidepressants
The risk of side effects increases if you are taking medicines such as certain antidepressants. Durfenta can interact with these medicines and you may experience changes in your mental state such as agitation, seeing, feeling, hearing or smelling things that are not there (hallucinations) and other effects such as changes in blood pressure, rapid heartbeat, high body temperature, overactive reflexes, lack of coordination, muscle stiffness, nausea, vomiting and diarrhea (these could be signs of a serotonin syndrome). If they are used together, your doctor may want to monitor you closely to detect these side effects, especially when starting treatment or when changing the dose of the medicine.
Using Durfenta with central nervous system depressants, including alcohol and some narcotics
Using Durfenta and medicines with a sedating effect, such as benzodiazepines or related medicines, increases the risk of drowsiness, breathing difficulties (respiratory depression), coma and can be life-threatening. Therefore, concomitant use should only be considered when other treatment options are not possible.
However, if your doctor prescribes Durfenta together with sedating medicines, the dose and duration of concomitant treatment should be limited by your doctor.
Please inform your doctor about any sedating medicine you are taking, and strictly follow the dose recommended by your doctor. It may be useful to inform friends or family members about the signs and symptoms described above. Contact your doctor if you experience any of these symptoms.
Do not drink alcohol while using Durfenta, unless you have talked to your doctor first.
Use in athletes
Athletes are informed that this medicine contains a component that can result in a positive doping test.
Operations
If you are going to have an operation, tell your doctor or dentist that you are using Durfenta.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Durfenta should not be used during pregnancy, unless you have discussed it with your doctor.
Durfenta should not be used during labor, as the medicine can affect the breathing of the newborn baby.
Long-term use of Durfenta during pregnancy can cause withdrawal symptoms in your newborn baby, such as loud crying, irritability, convulsions, poor feeding and diarrhea, which can be life-threatening if not recognized and treated. Talk to your doctor immediately if you think your baby may have withdrawal symptoms.
Do not use Durfenta if you are breastfeeding. You should not breastfeed for 3 days after removing the Durfenta patch. This is because the medicine can pass into breast milk.
Driving and using machines
Durfenta can affect your ability to drive and use machines or tools, as it can make you sleepy or dizzy. If this happens, do not drive or use machines or tools. Do not drive while using this medicine until you know how it affects you.
Talk to your doctor or pharmacist if you are unsure whether it is safe for you to drive while using this medicine.
3. How to use Durfenta
Follow your doctor's administration instructions for this medication exactly. If in doubt, consult your doctor or pharmacist again.
Your doctor will decide which concentration of Durfenta is most suitable for you, taking into account the intensity of your pain, your general condition, and the type of pain treatment you have received so far.
Before starting treatment and regularly during treatment, your doctor will also explain what you can expect from using Durfenta, when and for how long you should use it, when you should contact your doctor, and when you should stop using it (see also section 2, "Symptoms of withdrawal from stopping Durfenta").
How to apply and change patches
- Each patch contains enough medication for 3 days (72 hours).
- You should change the patch every three days, unless your doctor has told you otherwise.
- Always remove the used patch beforeapplying a new one.
- Always change the patch at the same timeof day every 3 days (72 hours).
- If you are using more than one patch, change them all at the same time.
- Write down the day of the week, date, and time each time you apply a patch to remember when you need to change it.
- The following table shows when you should change the patch:
Apply the patch on | Change the patch on | |
Monday | ? | Thursday |
Tuesday | ? | Friday |
Wednesday | ? | Saturday |
Thursday | ? | Sunday |
Friday | ? | Monday |
Saturday | ? | Tuesday |
Sunday | ? | Wednesday |
Where to apply the patch
Adults
- Apply the patch to a flat area of the torso or arm (never over a joint).
Children
- Always apply the patch to the upper back so that your child has difficulty reaching or removing it.
- Check occasionally to ensure the patch is still attached to the skin.
- It is essential that the child does not remove the patch and put it in their mouth, as this could be life-threatening or fatal.
- Keep your child under close observation for 48 hours after:
- Applying the first patch
- Applying a patch with a higher dose
- The patch may take some time to reach its maximum effect. Therefore, your child may need other pain relievers until the patch is effective. Your doctor will explain this to you.
Adults and children:
Do not apply the patch to
- The same site twice in a row.
- Areas that move a lot (joints), irritated skin, or skin with wounds.
- Areas of skin with a lot of hair. If there is hair, do not shave it (the skin becomes irritated with shaving). Instead, cut it as close to the skin as possible.
How to apply the patch
Step 1: Prepare the skin
- Before applying the patch, check that the skin is completely dry, clean, and fresh
- If you need to wash the skin, use only cold water
- Do not use soap or other cleaners, creams, moisturizers, oils, or talcum powder before applying the patch
- Do not apply the patch immediately after a hot bath or shower
Step 2: Open the envelope
- Each patch is sealed in its own envelope
- Open the envelope by tearing or cutting it at the notch, marked with an arrow
- Gently tear or completely cut the edge of the envelope (if using scissors, make the cut along the sealed edge to avoid damaging the patch)
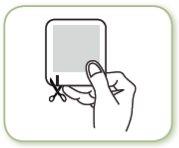
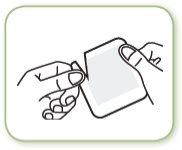
- Hold the two parts of the open envelope and pull to separate them
- Remove the patch and use it immediately
- Save the empty envelope to dispose of the used patch later
- Use each patch only once
- Do not remove the patch from its envelope until you are ready to use it
- Check that the patch is not damaged
- Do not use the patch if it is divided or cut or if it has any damage
- Never divide or cut the patch
Step 3: Peel and press
- Make sure the patch will be covered by loose clothing and not stuck under tight or elastic fabric.
- Remove the shiny plastic protective layer that covers the printed side of the patch.
- Carefully peel one corner of the patch from the shiny plastic protective layer that covers the adhesive part of the patch. Try not to touch the adhesive part of the patch.
- Press this adhesive part of the patch onto the skin with the palm of your hand.
- Hold it pressed for at least 30 seconds. Make sure the patch is well adhered, especially around the edges.
Step 4: Dispose of the patch
- As soon as you remove the patch, fold it firmly in half so that the adhesive side of the patch sticks to itself
- Put it back in the original envelope and throw it away following the instructions of your pharmacist
- Keep used patches out of sight and reach of children; although they are used, patches contain medication that can harm children and even be fatal
Step 5: Wash your hands
- Always wash your hands with water only after handling patches
More information on using Durfenta
Daily activities during patch use
- Patches are water-resistant
- You can shower or bathe with the patch on, but do not rub it
- If your doctor agrees, you can exercise or play sports with the patch on
- You can also swim with the patch on, but:
- Do not use hot tubs or whirlpools
- Do not cover the patch with tight or elastic fabrics
- When wearing the patch, do not expose it to direct heat, such as heating pads, electric blankets, hot water bottles, waterbeds, or heat lamps or tanning beds. Do not sunbathe or take long hot baths and do not use saunas. If you do, you may increase the amount of medication released from the patch.
How long do patches take to work?
- The first patch may take some time to reach its maximum effect.
- Your doctor may give you additional pain relievers during the first few days.
- Afterwards, the patch should help relieve pain continuously, so you may be able to stop taking other pain relievers. However, your doctor may prescribe additional pain relievers from time to time.
How long will you need to use patches?
- Durfenta patches are indicated for prolonged pain. Your doctor will tell you how long you can expect to use the patches.
If your pain worsens
- If your pain worsens suddenly after applying the last patch, check the patch. If it is no longer sticking well or has come off, you should replace the patch (see also the section If the patch comes off).
- If your pain worsens over time while using the patches, your doctor may try patches with a higher dose or prescribe additional pain relievers (or both)
- If increasing the patch dose does not help, your doctor may decide to stop using the patches.
If you use too many patches or a patch with the wrong dose
If you have applied too many patches or a patch with the wrong dose, remove them and contact your doctor immediately, go to the hospital, or call the Toxicology Information Service, phone 91 562 04 20, indicating the medication and the amount applied.
Signs of overdose include breathing problems or weak breathing, tiredness, excessive drowsiness, inability to think clearly or walk or talk normally, and dizziness, dizziness, or confusion. An overdose can also cause a brain disorder known as toxic leukoencephalopathy.
If you forget to change the patch
- If you forget to change the patch, change it as soon as you remember and write down the day and time. Change the patch again after 3 days (72 hours), as usual.
- If you are significantly delayed, you should talk to your doctor, as you may need additional pain relievers, but do notapply additional patches.
If the patch comes off
- If the patch falls off before it is due to be changed, apply a new one immediately and write down the day and time. Choose a new skin area:
- On the torso or arm
- On the upper back of your child
- Tell your doctor about this and leave the patch on for another 3 days (72 hours)or for the time your doctor indicates, before changing the patch in the usual way
- If patches tend to fall off, consult your doctor, pharmacist, or nurse
If you want to stop using patches
- Do not stop using this medication abruptly. If you want to stop using this medication, talk to your doctor first. Your doctor will tell you how to do it, usually by gradually reducing the dose so that the unpleasant effects of withdrawal are minimal. See also section 2, "Withdrawal symptoms when stopping Durfenta".
- If you stop using patches, do not start using them again without asking your doctor first. You may need a different dose when you restart treatment.
If you have any doubts about using this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medications, this medication can cause side effects, although not everyone will experience them.
If you, your partner, or caregiver notice any of the following signs in the person wearing the patch, remove the patch and call a doctor or go to the nearest hospital immediately. You may need urgent medical treatment.
- Unusual drowsiness, slower or weaker breathing than expected.
Follow the above recommendations and make the person wearing the patch move and talk as much as possible. In very rare cases, these breathing difficulties can be life-threatening or even fatal, especially in people who have not used strong opioid pain relievers before (such as Durfenta or morphine). (Uncommon, may affect up to 1 in 100 people).
- Sudden swelling of the face or throat, severe irritation, redness, or blisters on the skin. These can be signs of a severe allergic reaction. (Frequency cannot be estimated from available data).
- Seizures (convulsions). (Uncommon, may affect up to 1 in 100 people).
- Decreased level of consciousness or loss of consciousness. (Uncommon, may affect up to 1 in 100 people).
The following side effects have also been reported
Very common (may affect more than 1 in 10 patients)
- Nausea, vomiting, constipation
- Feeling drowsy (drowsiness)
- Feeling dizzy
- Headache.
Common (may affect up to 1 in 10 patients)
- Allergic reaction
- Lack of appetite
- Difficulty sleeping
- Depression
- Feeling anxious or confused
- Seeing, feeling, hearing, or smelling things that are not there (hallucinations)
- Tremors or muscle spasms
- Strange sensation on the skin, such as tingling or prickling (paresthesia)
- Feeling that everything is spinning (vertigo)
- Fast or irregular heartbeats (palpitations, tachycardia)
- Increased blood pressure
- Feeling short of breath (dyspnea)
- Diarrhea
- Dry mouth
- Stomach pain or indigestion
- Excessive sweating
- Itching, rash, or redness of the skin
- Difficulty urinating or emptying the bladder completely
- Extreme fatigue, weakness, or general malaise
- Feeling cold
- Swelling of the hands, ankles, or feet (peripheral edema)
Uncommon (may affect up to 1 in 100 patients)
- Feeling agitated or disoriented
- Feeling extremely happy (euphoria)
- Decreased sensation or sensitivity, especially in the skin (hypoesthesia)
- Memory loss
- Blurred vision
- Slow heart rate (bradycardia) or low blood pressure
- Bluish color of the skin due to decreased oxygen in the blood (cyanosis)
- Lack of intestinal contractions (ileus)
- Skin rash with itching (eczema), allergic reaction, or other skin disorders where the patch is applied
- Flu-like illness
- Feeling of changed body temperature
- Fever
- Muscle contraction
- Difficulty getting or maintaining an erection (impotence) or problems having sex
- Difficulty swallowing.
Rare (may affect up to 1 in 1,000 patients)
- Pupil constriction (miosis)
- Occasional interruption of breathing (apnea)
Frequency not known (cannot be estimated from available data)
- Lack of male sex hormones (androgen deficiency)
- Delirium (symptoms may include a combination of agitation, restlessness, disorientation, confusion, fear, seeing or hearing things that are not really there, sleep disorders, nightmares)
- You may become dependent on Durfenta (see section 2).
You may notice rashes, redness, or mild itching of the skin at the patch application site. It is usually mild and disappears after removing the patch. If it does not, or if the patch irritates your skin a lot, tell your doctor.
Repeated use of patches can cause the medication to lose effectiveness (you get used to it or become more sensitive to pain) or can cause dependence.
If you switch from another pain reliever to Durfenta or if you stop using Durfenta abruptly, you may experience withdrawal symptoms, such as dizziness, feeling sick, diarrhea, anxiety, or tremors. Tell your doctor if you notice any of these effects.
There have also been reports of newborn babies experiencing withdrawal symptoms after their mothers used Durfenta for a prolonged period during pregnancy.
Reporting side effects:
If you experience any side effects, consult your doctor or pharmacist, even if they are possible side effects not listed in this leaflet. You can also report them directly through the Spanish Medicines Monitoring System for Human Use: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medication.
5. Storage of Durfenta
Where to store patches
Keep all patches (used and unused) out of sight and reach of children.
Store this medication in a safe and protected place, where others cannot access it. This medication can cause serious harm and even be fatal if used accidentally or intentionally by people who have not been prescribed it.
How long can you store Durfenta
Do not use this medication after the expiration date shown on the box and envelope. The expiration date is the last day of the month indicated. If patches are expired, take them to the pharmacy.
Do not store above 25 °C.
Store in the original envelope to protect it from moisture.
How to dispose of used patches or patches you no longer use
Accidental exposure of another person to used and unused patches, especially children, can result in a fatal outcome.
Used patches should be folded firmly in half so that the adhesive side of the patch sticks to itself. Then, they should be thrown away safely by placing them in the original envelope and keeping them out of sight and reach of others, especially children, until they are disposed of safely.
Medications should not be thrown down the drain or into the trash. Deposit the containers and medications you no longer need at the SIGRE collection point in the pharmacy. If in doubt, ask your pharmacist how to dispose of the containers and medications you no longer need. This way, you will help protect the environment.
6. Container Content and Additional Information
Durfenta Composition
- The active ingredient is fentanyl.
Each Durfenta 12 micrograms/hour transdermal patch contains 1.375 mg of fentanyl in a 5 cm2 surface area, releasing 12.5 micrograms of fentanyl per hour.
Each Durfenta 25 micrograms/hour transdermal patch contains 2.75 mg of fentanyl in a 10 cm2 surface area, releasing 25 micrograms of fentanyl per hour.
Each Durfenta 50 micrograms/hour transdermal patch contains 5.5 mg of fentanyl in a 20 cm2 surface area, releasing 50 micrograms of fentanyl per hour.
Each Durfenta 75 micrograms/hour transdermal patch contains 8.25 mg of fentanyl in a 30 cm2 surface area, releasing 75 micrograms of fentanyl per hour.
Each Durfenta 100 micrograms/hour transdermal patch contains 11.0 mg of fentanyl in a 40 cm2 surface area, releasing 100 micrograms of fentanyl per hour.
- The other components are:
Coating layer:
Polyethylene terephthalate laminate with fluorocarbon release coating.
Support layer:
Pigmented polyethylene terephthalate/ethyl vinyl acetate copolymer laminate.
Adhesive layer to the matrix:
Silicone adhesive (dimeticone, silicate resin).
Dimeticone.
Rate-controlling membrane:
Ethyl vinyl acetate copolymer laminate.
Adhesive layer to the skin:
Silicone adhesive (dimeticone, silicate resin).
Dimeticone.
Protective layer:
Polyethylene terephthalate laminate with fluorocarbon release coating.
Printing inks:
Beige and orange or red or green or blue or gray ink
Product Appearance and Container Content
The Durfenta transdermal patch is rectangular with rounded corners, printed on its backing with:
- diagonal beige stripes with "Fentanyl" repeated in orange font alternating with diagonal orange stripes with "12 μg/h" repeated in beige font.
- diagonal beige stripes with "Fentanyl" repeated in red font alternating with diagonal red stripes with "25 μg/h" repeated in beige font.
- diagonal beige stripes with "Fentanyl" repeated in green font alternating with diagonal green stripes with "50 μg/h" repeated in beige font.
- diagonal beige stripes with "Fentanyl" repeated in blue font alternating with diagonal blue stripes with "75 μg/h" repeated in beige font.
- diagonal beige stripes with "Fentanyl" repeated in gray font alternating with diagonal gray stripes with "100 μg/h" repeated in beige font
Each patch has an adhesive backing that allows it to be stuck to the skin. The patch is covered by two large transparent protective films that are removed before application.
Durfenta is available in packs of 3, 4, 5, 8, 9, 10, 16, 19, or 20 transdermal patches.
Only some pack sizes may be marketed.
Marketing Authorization Holder and Manufacturer:
Lavipharm S.A.
Agias Marinas Street
GR-19002 Peania, Attica,
Greece
For further information about this medicinal product, please contact the local representative of the marketing authorization holder:
Especialidades Farmacéuticas Centrum, S.A.,
Sagitario Street, 14, 03006 Alicante, Spain
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Austria Lafene 12/25/50/75/100 Mikrogramm/h transdermales Pflaster
Spain Durfenta 12/25/50/75/100 micrograms/hour transdermal patch EFG
Ireland Fentadur 12/25/50/75/100 microgram/hour transdermal patch
Denmark Lafene
Luxembourg Recorfen 25/50/75/100 μg/h transdermales Pflaster // dispositif transdermique
Portugal Fentanilo Lavipharm 12, 25, 50, 75, 100 μg/h transdermal system
Romania Fentanyl Lavipharm 12, 25, 50, 75, 100 micrograme/h transdermic patch
Date of last revision of this leaflet:March 2024
Detailed and updated information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price58.51 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to DURFENTA 100 micrograms/HOUR TRANSDERMAL PATCHESDosage form: TRANSDERMAL PATCH, 12 MCG/HActive substance: fentanylManufacturer: Aristo Pharma Iberia S.L.Prescription requiredDosage form: BUCCAL/SUCKING TABLET, 1200 microgramsActive substance: fentanylManufacturer: Ferrer Internacional S.A.Prescription requiredDosage form: BUCCAL/SUCKING TABLET, 1600 microgramsActive substance: fentanylManufacturer: Ferrer Internacional S.A.Prescription required
Online doctors for DURFENTA 100 micrograms/HOUR TRANSDERMAL PATCHES
Discuss questions about DURFENTA 100 micrograms/HOUR TRANSDERMAL PATCHES, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















