
DEZACOR 22.75 mg/ml ORAL SUSPENSION DROPS

How to use DEZACOR 22.75 mg/ml ORAL SUSPENSION DROPS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Leaflet: information for the user
Dezacor 22.75 mg/ml oral suspension drops
deflazacort
Read this leaflet carefully before starting to take this medication, as it contains important information for you.
- Keep this leaflet, as you may need to read it again.
- If you have any doubts, consult your doctor or pharmacist.
- This medication has been prescribed to you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, consult your doctor or pharmacist, even if they are not listed in this leaflet. See section 4.
Contents of the leaflet
- What is Dezacor and what is it used for
- What you need to know before taking Dezacor
- How to take Dezacor
- Possible side effects
- Storage of Dezacor
- Package contents and additional information
1. What is Dezacor and what is it used for
Dezacor is a medication belonging to a group of medications known as corticosteroids, which have anti-inflammatory and anti-allergic properties.
This medication can be used to treat diseases whose severity requires immediate systemic treatment with glucocorticoids. These include:
- Rheumatic and collagen diseases: treatment of rheumatoid arthritis and psoriatic arthritis when conservative treatments have been ineffective; polymyalgia rheumatica; acute rheumatic fever; systemic lupus erythematosus; severe dermatomyositis; polyarteritis nodosa; cranial arteritis and Wegener's granulomatosis.
- Skin diseases: bullous pemphigoid, generalized exfoliative dermatitis, erythema multiforme, erythema nodosum, and severe psoriasis.
- Allergic diseases: bronchial asthma refractory to conventional therapy.
- Pulmonary diseases: sarcoidosis with pulmonary involvement, extrinsic allergic alveolitis (organic dust pneumoconiosis), and idiopathic pulmonary fibrosis.
- Inflammatory eye diseases: choroiditis, chorioretinitis, iritis, and iridocyclitis.
- Hematological diseases: idiopathic thrombocytopenia, hemolytic anemias, and palliative treatment of leukemias and lymphomas.
- Diseases of the digestive system and liver: ulcerative colitis, Crohn's disease, and chronic active hepatitis.
- Kidney diseases: nephrotic syndrome.
2. What you need to know before taking Dezacor
Do not take Dezacor
- If you are allergic (hypersensitive) to deflazacort or any of the other components of this medication (listed in section 6).
- If you are receiving live virus vaccines
- If you have a generalized infection without specific treatment
- If you have a stomach ulcer.
- If you have bacterial infections (active tuberculosis) and viral infections (herpes simplex ocular, herpes zoster, chickenpox) or generalized fungal infections.
- If you are in the pre- or post-vaccination period.
Warnings and precautions
Consult your doctor before starting to take this medication.
- It is essential that your doctor knows about all the diseases you have or have had before they can advise you on this treatment. Especially, you must inform them about cardiovascular diseases (heart failure, high blood pressure), blood clotting disorders (thrombosis, embolism), digestive or intestinal diseases (stomach ulcers, intestinal inflammation, chronic diarrhea), severe liver or kidney diseases, diabetes, osteoporosis, behavioral disorders (mood changes, insomnia), epilepsy, glaucoma, thyroid gland insufficiency, muscle weakness, and certain acute or chronic infections. Previous or existing history of severe affective disorders or in first-degree relatives (depressive or manic-depressive illnesses and psychoses).
- The use of corticosteroids whose duration exceeds that of a short-term replacement or emergency treatment is contraindicated in the following cases: peptic ulcer, bacterial and viral infections such as active tuberculosis, herpes simplex ocular, herpes zoster (viremic phase), as well as in systemic mycotic infections and in the pre- and post-vaccination period.
- In prolonged treatments, ocular alterations may appear, so your doctor may advise you to visit an ophthalmologist periodically.
- Contact your doctor if you experience blurred vision or other visual disturbances.
- It may be necessary to adjust the dose of corticosteroids in special situations (surgery, infections, and others). Inform your doctor if you present any of these processes during treatment with Dezacor.
- Treatment with deflazacort may cause irregular menstruation and leukocytosis.
- You should be especially careful to avoid exposure to measles and chickenpox; consult your doctor immediately in case of exposure.
- In children, prolonged use of this medication can stop their growth and development.
- Consult a doctor if you experience worrying psychological symptoms, especially if you suspect a depressive mood or suicidal thoughts. You should be alert to possible psychiatric disorders that may appear during or immediately after reducing or withdrawing the medication dose, although such reactions have been reported with low frequency.
After a long treatment with Dezacor, it should be gradually discontinued. Do not discontinue this medication without consulting your doctor first.
Use in athletes
Patients should be warned that this medication contains deflazacort, which can produce a positive result in doping tests.
Other medications and Dezacor
Inform your doctor or pharmacist if you are taking, have recently taken, or may need to take any other medication.
Some medications can increase the effects of Dezacor 22.75 mg/ml oral suspension drops, so your doctor will closely monitor you if you are taking these medications (including some for HIV: ritonavir, cobicistat).
In particular, inform your doctor or pharmacist if you are taking any of the medications listed below, as Dezacor may interact with them.
- Pain or inflammation medications.
- Diabetes medications: as a dose change may be necessary.
- Antihypertensives and diuretics: as a dose change may be necessary.
- Antibiotics (rifampicin): as they can decrease the effect of Dezacor.
- Estrogens or oral contraceptives: as the effect of Dezacor may be increased.
- Muscle relaxants: as the relaxing effect may be prolonged.
- Anticholinesterase medications used in myasthenia gravis.
- Medications for the treatment of heart failure or coagulation disorders.
- Vaccines and toxoids: as corticosteroids decrease the immune response.
- Medications for epilepsy and those used in psychiatric treatments (carbamazepine, phenytoin, phenobarbital): as they can decrease the effect of Dezacor.
- Anticoagulant medications: as corticosteroids can increase or decrease their effects.
- Antacids: as they can reduce the bioavailability of Dezacor.
Do not take any of these medications at the same time as Dezacor without consulting your doctor.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medication. The use of medications during pregnancy can be hazardous to the embryo or fetus and should be monitored by your doctor.
Human experience is limited; therefore, deflazacort will only be used in cases where the risk-benefit assessment advises its use.
Dezacor is excreted in breast milk, so its use is not recommended during breastfeeding. The use of deflazacort requires weighing the benefits of breastfeeding against the potential risks.
Driving and using machines
No data are available, although it is advisable that, until the response to treatment is satisfactory, you do not perform tasks that require special attention, such as driving vehicles or operating hazardous machinery.
Dezacor 22.75 mg/ml oral suspension drops contain sorbitol (E-420), sodium, and benzyl alcohol.
This medication contains sorbitol. If your doctor has told you that you have an intolerance to certain sugars, consult with them before taking this medication.
This medication contains less than 23 mg of sodium (1 mmol) per ml; i.e., it is essentially "sodium-free".
This medication contains 10.45 mg of benzyl alcohol (0.01 ml) per ml of product. Benzyl alcohol can cause allergic reactions. Large amounts of benzyl alcohol can accumulate in the body and cause adverse effects (metabolic acidosis), especially in pregnant or breastfeeding women and in patients with liver or kidney failure. In these cases, consult your doctor or pharmacist.
Benzyl alcohol has been linked to the risk of serious adverse effects, including respiratory problems ("gasping syndrome") in children.
This medication should not be administered to newborns (up to 4 weeks of age) unless recommended by your doctor.
This medication should not be used for more than one week in children under 3 years of age, unless recommended by your doctor.
3. How to take Dezacor
Follow your doctor's instructions for taking this medication exactly. If in doubt, consult your doctor or pharmacist again.
Your doctor will determine the daily dose. The dosage is individual for each patient, depending on the type and severity of their disease, as well as their response to treatment.
In adults, the dose can range from 6 to 90 mg per day, and in children from 0.25 to 1.5 mg/kg. It is essential that you understand your doctor's instructions for taking the medication and, if in doubt, do not hesitate to consult them.
In special situations (stress, significant infections, severe trauma, or surgery), it may be necessary to adjust the dose. Consult your doctor to explain the procedure in these cases.
Instructions for correct administration
This medication is administered orally. The bottle should be shaken before use.
The drops to be administered can be diluted immediately before taking in sugared water or non-carbonated beverages.
Use of the package
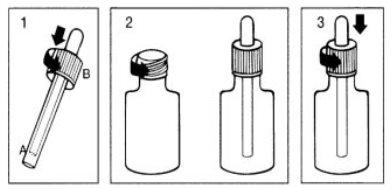
- To release the dropper from its protection, hold A and pull B upwards at the same time.
- Remove the metal cap from the bottle and place and screw on the dropper.
- To open the bottle containing the dropper, press the cap firmly and unscrew at the same time.
CHILD-RESISTANT CLOSURE.
Your doctor will indicate the duration of the treatment. Do not discontinue it before without authorization and never do so abruptly.
After a prolonged treatment, the administration of this medication should never be interrupted abruptly. Your doctor will indicate how to gradually decrease the dose. It is also important that you remain in contact with your doctor at the end of the treatment so that they can act in case of symptom recurrence.
If you take more Dezacor than you should
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone 91 562 04 20, indicating the medication and the amount ingested, and go to a hospital for appropriate treatment.
If you forget to take Dezacor
Do not take a double dose to make up for forgotten doses.
If you interrupt treatment with Dezacor
Prolonged treatments that are interrupted abruptly can cause: fever, discomfort, and muscle and joint pain.
If you have any other doubts about the use of this medication, ask your doctor or pharmacist.
4. Possible side effects
Like all medications, Dezacor can cause side effects, although not everyone will experience them.
In short-term treatments, this medication is well tolerated, and side effects are rare. However, in prolonged treatments, the following have been observed:
Common: may affect up to 1 in 10 people
- Weight gain.
Uncommon: may affect up to 1 in 100 people
- Allergy or hypersensitivity to deflazacort.
- Pain or discomfort in the abdomen, stomach ulcers, bleeding, nausea, indigestion.
- Headache, dizziness.
- Depressed or unstable mood.
- Behavioral changes, mood swings (depression, euphoria).
- Hirsutism (excessive hair growth in women), stretch marks, and acne.
- Suppression of the hypothalamic-pituitary-adrenal axis (can cause impaired response to stress and inadequate defense against infections), Cushingoid face (moon face).
- Elevation of blood glucose (with onset or worsening of diabetes), sodium and water retention (with increased blood pressure), potassium loss in the urine when administered with beta-agonist medications and xanthines (salbutamol, isoprenaline, fluticasone, theophylline).
- Increased susceptibility to infections due to reduced immune system activity.
- Osteoporosis, vertebral and long bone fractures.
- Edema (swelling caused by fluid accumulation in body tissues).
Rare: may affect up to 1 in 1,000 people
- Bruises.
- Muscle mass loss.
Frequency not known (cannot be estimated from available data):
- Leukocytosis (increase in the number of white blood cells in the blood).
- Clot formation, particularly in patients with conditions associated with a higher tendency to develop thrombi.
- Perforation of peptic ulcer, acute pancreatitis (especially in children), candidiasis (fungal infection of the skin and mucous membranes).
- Restlessness, increased intracranial pressure in children (usually after treatment withdrawal), worsening of epilepsy.
- Irritability, euphoria, suicidal thoughts.
- Mania, delirium, hallucinations, worsening of schizophrenia.
- Anxiety, sleep disorders, and cognitive dysfunction (alteration in higher brain functions such as language, orientation, memory, interpretation of reality, or social behavior).
- Blurred vision, increased intraocular pressure, glaucoma, edema of the optic disc, cataracts (especially in children), chorioretinopathy (retinal alteration that can cause vision changes), corneal thinning, worsening of ocular infections caused by viruses or fungi.
- Thinning of the skin, appearance of small blood vessels in the skin (spider veins).
- Heart failure, hypertrophic cardiomyopathy in premature newborns.
- Growth retardation in children.
- Increased protein and calcium loss, increased appetite.
- Avascular necrosis of bone (bone destruction due to insufficient blood supply), tendinitis, and tendon rupture when taken with quinolone-type antibiotics (ciprofloxacin, ofloxacin, levofloxacin), muscle weakness or alterations.
- Menstrual irregularities.
- Impaired wound healing.
- A too rapid reduction in the dose of this medication after prolonged treatment can lead to acute adrenal insufficiency (a potentially life-threatening condition that occurs when there is a lack of cortisol), hypotension, and death.
The use of Dezacor together with muscle relaxant medications, especially when administered at high doses and for extended periods, can cause severe muscle alterations.
During treatment with this medication, your tendency to infections may increase, so if you notice any symptoms of disease that could be related to taking it, you should contact your doctor.
Reporting side effects
If you experience any type of side effect, consult your doctor or pharmacist, even if it is a possible side effect not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medications: https://www.notificaram.es/. By reporting side effects, you can contribute to providing more information on the safety of this medication.
5. Storage of Dezacor
Keep out of sight and reach of children.
No special storage conditions are required.
Do not use this medication after the expiration date shown on the box after CAD. The expiration date is the last day of the month indicated.
Discard the package 1 month after opening.
Medications should not be thrown away in drains or trash. Deposit the packages and medications you no longer need in the SIGRE point of the pharmacy. If in doubt, ask your pharmacist how to dispose of packages and medications you no longer need. This will help protect the environment.
6. Container Content and Additional Information
Composition of Dezacor
The active ingredient is deflazacort. Each ml of suspension contains 22.75 mg of deflazacort or each drop of suspension contains 1 mg of deflazacort.
The other components are: sorbitol solution 70%, sodium carboxymethylcellulose, aluminum and magnesium silicate, polysorbate 80, benzyl alcohol, sucralose, tropical fruit flavor, citric acid monohydrate, sodium hydroxide, and purified water.
Appearance of Dezacor and Container Content:
Homogeneous suspension of a whitish color.
It is presented in 20 ml topaz glass bottles with an aluminum cap, including a glass dropper. The container content is 13 ml.
Other Presentations
Dezacor is also marketed in 6 mg and 30 mg tablets.
Marketing Authorization Holder
Faes Farma, S.A.
Autonomia Etorbidea, 10
48940 Leioa (Bizkaia)
Spain
Manufacturer
Faes Farma, S.A.
Maximo Agirre Kalea, 14
48940 Leioa (Bizkaia)
Spain
Or
Faes Farma, S.A.
Bizkaia Science and Technology Park
Ibaizabal Bidea, Building 901
48160 Derio (Bizkaia)
Spain
Date of the Last Revision of this Prospectus: April 2025
Detailed and updated information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price3.9 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to DEZACOR 22.75 mg/ml ORAL SUSPENSION DROPSDosage form: TABLET, 30 mgActive substance: deflazacortManufacturer: Faes Farma S.A.Prescription requiredDosage form: TABLET, 6 mgActive substance: deflazacortManufacturer: Faes Farma S.A.Prescription requiredDosage form: TABLET, 30 mgActive substance: deflazacortManufacturer: Laboratorios Alter S.A.Prescription required
Online doctors for DEZACOR 22.75 mg/ml ORAL SUSPENSION DROPS
Discuss questions about DEZACOR 22.75 mg/ml ORAL SUSPENSION DROPS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions











