
CAYSTON 75 mg POLVO Y DISOLVENTE PARA SOLUCION PARA INHALACION POR NEBULIZADOR

Cómo usar CAYSTON 75 mg POLVO Y DISOLVENTE PARA SOLUCION PARA INHALACION POR NEBULIZADOR
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Cayston 75 mg polvo y disolvente para solución para inhalación por nebulizador
Aztreonam
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Cayston y para qué se utiliza
- Qué necesita saber antes de empezar a usar Cayston
- Cómo usar Cayston
- Posibles efectos adversos
- Conservación de Cayston
- Contenido del envase e información adicional
1. Qué es Cayston y para qué se utiliza
Cayston contiene el principio activo aztreonam. Cayston es un antibiótico que se utiliza para el tratamiento de las infecciones pulmonares crónicas causadas por la bacteria Pseudomonas aeruginosa en pacientes de 6 o más años de edad con fibrosis quística. La fibrosis quística, también conocida como mucoviscidosis, es una enfermedad hereditaria potencialmente mortal, que afecta a las glándulas mucosas de los órganos internos, especialmente a los pulmones, pero también al hígado, al páncreas y al aparato digestivo. En los pulmones, la fibrosis quística causa una congestión con moco pegajoso. Esto produce dificultad para respirar.
2. Qué necesita saber antes de empezar a usar Cayston
No use Cayston
- si es alérgico al aztreonam o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6).
Advertencias y precauciones
Consulte a su médico antes de empezar a usar Cayston:
- si es alérgico a cualquier otro antibiótico(como penicilinas, cefalosporinas y/o carbapenemos)
- si no tolera o siente opresión en el pecho al tomar otros medicamentos inhalados
- si tiene problemas en los riñones
- si ha expectorado sangreen alguna ocasión
- si ha tenido pruebas de función pulmonar bajaen alguna ocasión.
Si se encuentra en alguna de las circunstancias anteriores, informe a su médicoantes de usar Cayston.
Dado que es un medicamento inhalado, Cayston puede provocarle tos, lo que podría causar una expectoración de sangre. Si ha expectorado sangre en alguna ocasión, solo debe usar Cayston si su médico cree que el beneficio de tomar este medicamento supera al riesgo de expectorar sangre.
Durante el tratamiento con Cayston puede presentar una disminución temporal de las pruebas de función pulmonar, pero, por regla general, no es un efecto que perdure.
Niños
Cayston no debe administrarse a niños menores de 6 años.
Otros medicamentos y Cayston
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o podría tener que utilizar cualquier otro medicamento.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico antes de utilizar este medicamento.
No existen datos clínicos sobre el uso de Cayston en mujeres embarazadas, por lo tanto, no debe usar Cayston durante el embarazo, a menos que lo haya discutido específicamente con su médico.
Si planea dar el pecho a su bebé, consulte a su médico antes de usar Cayston. Puede dar el pecho durante el tratamiento con Cayston, ya que la cantidad de medicamento que probablemente pase a su hijo durante la lactancia será extremadamente pequeña.
Conducción y uso de máquinas
No es de esperar que Cayston afecte a su capacidad para conducir o usar máquinas.
3. Cómo usar Cayston
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
La dosis recomendada es:
- Use Cayston 3 veces al día en ciclos repetidos de 28 días de terapia seguidos de 28 días sin terapia con Cayston.Cada una de las tres dosis debe administrarse mediante inhalación con un intervalo de separación de al menos cuatro horas, utilizando un dispositivo nebulizador de mano Altera. Puede utilizar un eBase Controller o una unidad de mando eFlow rapid con el dispositivo de mano Altera.
- Cada dosis consiste en un vial de Cayston que se mezcla con el contenido de la ampolla de disolvente. Es necesario mezclar Cayston con un disolvente antes de inhalarse utilizando el nebulizador Altera.
Introduzca la solución Cayston preparada en el dispositivo nebulizador de mano Altera (ver a continuación). Cada tratamiento tarda alrededor de 2 a 3 minutos en inhalarse.
Utilice un broncodilatador antes de cada dosis de Cayston. Los broncodilatadores de acción corta pueden usarse entre 15 minutos y 4 horas antes de cada dosis de Cayston, y los de acción prolongada, entre 30 minutos y 12 horas antes.
Si está utilizando otras terapias por vía inhalatoria para tratar la fibrosis quística, el orden de uso recomendado es el siguiente:
- broncodilatador
- mucolíticos (un medicamento que ayuda a disolver el espeso moco producido en los pulmones) y finalmente:
- Cayston.
No mezcle Cayston con ningún otro medicamentoen el dispositivo nebulizador de mano Altera.
- No introduzca otros medicamentos en el dispositivo nebulizador de mano Altera.
- No introduzca aztreonam para administración intravenosa (inyectable) en el dispositivo nebulizador de mano Altera. El aztreonam intravenoso no es adecuado para la inhalación.
Cómo administrar Cayston utilizando el dispositivo nebulizador de mano Altera
Necesitará los siguientes elementos:
- Un vial de color ámbar de Cayston con una cápsula de cierre azul.
- Una ampolla de plástico de disolvente (cloruro de sodio al 0,17% p/v). La información que aparece en la ampolla de disolvente se presenta en inglés solamente (ver sección 6).
- Un dispositivo nebulizador de mano Altera con un generador de aerosol Altera conectado a una unidad de mando eFlow de tipo 178 (eFlow rapid) o de tipo 678 (eBase Controller).
Debe utilizar el dispositivo nebulizador de mano Altera específico de Cayston, con el generador de aerosol Altera. No intente utilizar Cayston con ningún otro tipo de dispositivo nebulizador de mano (incluso el dispositivo de mano eFlow rapid).
Compruebe que el nebulizador funciona adecuadamenteantes de comenzar el tratamiento con Cayston. Lea atentamente las instrucciones de uso del fabricante suministradas con el sistema nebulizador Altera.
Preparación de Cayston para la inhalación
- No prepare Cayston hasta que esté listo para administrar la dosis.
- No utilice Cayston si observa que el envase ha sido manipulado.
- No utilice Cayston si se ha conservado fuera de la nevera durante más de 28 días.
- No utilice el disolvente o Cayston ya preparado si presenta un aspecto turbio o se observan partículas en la solución.
- Extraiga un vial de color ámbar de Cayston y una ampolla de disolventede la caja. Las ampollas de disolvente deben separarse tirando suavemente de ellas.
- Golpee suavemente el vial de color ámbarde Cayston, de modo que el polvo se deposite en el fondo. Esto ayuda a garantizar que la dosis administrada sea la correcta.
- Siga los pasos de A a D en la Figura 1 a continuación para abrir el vial de color ámbar:
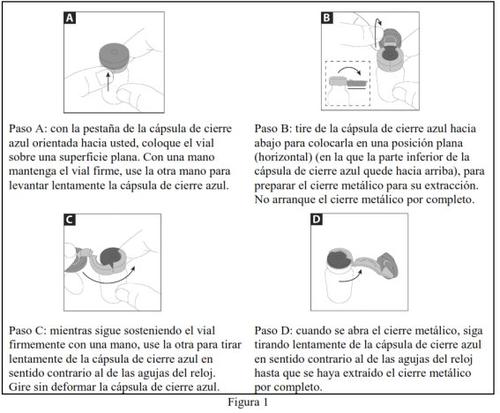
- Deseche el cierre metálico de forma segura. Saque cuidadosamente (pero no deseche todavía) el tapón de goma.
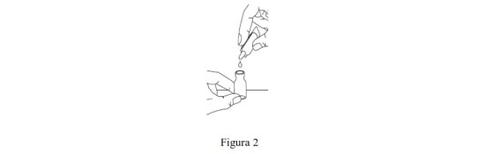
- Abra la ampolla de disolvente retorciendo su punta hasta extraerla. Apriete la ampolla para transferir su contenido íntegro al vial (Figura 2). A continuación, agite suavemente el vial con un movimiento circular hasta que el polvo se haya disuelto por completo y el líquido tenga un aspecto claro.
Es mejor utilizar Cayston inmediatamente después de preparar la solución.Pero, si no puede usar la dosis preparada de forma inmediata, vuelva a colocar el tapón al vial y guárdelo en la nevera. Utilice la solución preparada en un plazo máximo de 8 horas.
Preparación del nebulizador Altera para la administración de Cayston
- Asegúrese de que el dispositivo nebulizador de mano Alterase encuentre apoyado sobre una superficie lisa y estable.
- Extraiga la tapa del contenedor del medicamentogirándola en el sentido contrario al de las agujas del reloj.
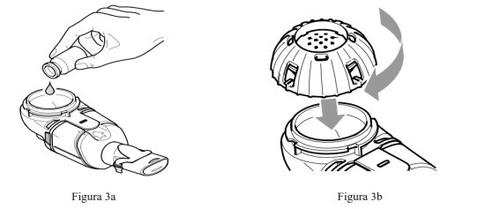
- Vierta todo el Cayston preparado del vialen el interior del contenedor del medicamento del dispositivo nebulizador de mano Altera (Figura 3a). Asegúrese de vaciar completamente el vial. Golpee suavemente el vial contra el lateral del contenedor del medicamento, si es necesario.
- Cierre el contenedor del medicamentoalineando las guías de la tapa del contenedor del medicamento con las ranuras del contenedor. Presione hacia abajo y gire la tapa en el sentido de las agujas del reloj hasta alcanzar el tope (Figura 3b).
Utilización del nebulizador Altera para la administración de Cayston
- Comience el tratamiento.Siéntese en posición relajada, con la espalda erguida. Sujete el dispositivo de mano en posición horizontal, introduzca la boquilla en su boca y cierre los labios a su alrededor (Figura 4).
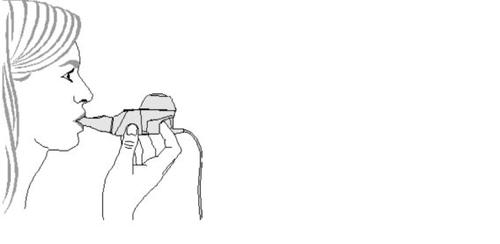
Mantenga el dispositivo de mano en posición horizontal.
- Apriete y mantenga presionada la tecla On/Offde la unidad de mando durante unos segundos. Oirá una señal acústica y la luz de estado se iluminará en verde.
- Al cabo de unos segundos, un aerosol en forma de neblina empezará a fluir hacia el interior de la cámara del nebulizador del dispositivo nebulizador de mano Altera. Si la neblina no comienza a fluir, consulte el manual del sistema Altera para obtener información.
- Respire con normalidad(inhale y expulse el aire) a través de la boquilla. Evite respirar por la nariz. Continúe inspirando y espirando cómodamente hasta terminar el tratamiento.
- Cuando se haya suministrado todo el medicamento, oirá un sonido que significa “tratamiento completo” (2 señales acústicas).
- Cuanto el tratamiento esté completo, abra la tapa del contenedor del medicamento para asegurarse de que éste se ha utilizado en su totalidad. Pueden quedar algunas gotas de medicamento en el contenedor una vez finalizado el tratamiento. Si quedan más que unas gotas de líquido, vuelva a colocar la tapa del contenedor del medicamento y reinicie el tratamiento.
- Una vez finalizado el tratamiento,desconecte la unidad de mando y aparte el dispositivo nebulizador de mano Altera para limpiarlo y desinfectarlo. Para obtener información detallada sobre los procedimientos de limpieza y desinfección, consulte las instrucciones de uso del fabricante suministradas con el dispositivo nebulizador de mano Altera.
¿Y si necesito interrumpir el tratamiento antes de terminarlo?
- Si, por cualquier motivo, tiene que interrumpir el tratamiento antes de terminarlo, apriete y mantenga presionada la tecla On/Off durante un segundo completo. Para reiniciar el tratamiento, apriete y mantenga presionada la tecla On/Off durante un segundo completo y reinicie el tratamiento.
Recambio del dispositivo nebulizador de mano Altera
El dispositivo nebulizador de mano Altera está diseñado para durar tres ciclos de 28 días de tratamiento con Cayston cuando se utiliza conforme a las instrucciones suministradas. Una vez transcurrido este tiempo, recambie su dispositivo nebulizador de mano Altera, incluido el generador de aerosol. Si nota que su funcionamiento ha sufrido cambios antes de transcurrido este tiempo (por ejemplo, si tarda más tiempo en generar la neblina, más de cinco minutos), consulte las instrucciones de uso del nebulizador Altera.
Si usa más Cayston del que debe
Si ha usado más Cayston del que debiera, consulte inmediatamente a un médico o farmacéutico.
Si olvidó usar Cayston
Si olvida una dosis, puede continuar administrándose las 3 dosis diarias siempre que deje un intervalo de al menos 4 horas de separación entre ellas. Si no puede dejar un espacio de 4 horas, omita la dosis que se saltó.
Si interrumpe el tratamiento con Cayston
No interrumpa el tratamiento con Cayston sin consultar antes con su médico.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Si le aparece un exantema, consulte a su médico inmediatamente,ya que puede significar que está sufriendo una reacción alérgica a Cayston.
Efectos adversos muy frecuentes (afectan a más de 1 de cada 10 pacientes)
- Tos
- Obstrucción nasal
- Silbidos respiratorios
- Dolor de garganta
- Sensación de falta de aire
- Fiebre. Puede ser más frecuente en los niños que en los adultos.
Efectos adversos frecuentes (afectan a entre 1 y 10 de cada 100 pacientes)
- Dificultad para respirar
- Molestias en el pecho
- Secreción nasal
- Expectoración de sangre
- Exantema
- Dolor en las articulaciones
- Resultados más bajos en las pruebas de función pulmonar
Efectos adversos poco frecuentes (afectan a entre 1 y 10 de cada 1.000 pacientes)
- Hinchazón de las articulaciones
Se han observado los siguientes efectos adversos después del uso de aztreonam inyectable, pero no después de la administración de Cayston: hinchazón de la cara, labios, lengua o garganta con dificultad para tragar o respirar, sudoración, irritación y descamación de la piel, erupción cutánea con picor, rubor, pequeñas manchas rojas y, muy rara vez, ampollas en la piel. Todos estos signos pueden indicar una reacción alérgica.
Informe a su médico si sufre cualquiera de estos efectos.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Cayston
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la etiqueta del vial, la ampolla de disolvente y el embalaje. La fecha de caducidad es el último día del mes que se indica.
Vial de polvo y ampolla de disolvente:
Conservar en nevera (entre 2°C y 8°C). Los viales sin abrir también pueden conservarse fuera de la nevera, pero a una temperatura inferior a 25°C durante un máximo de 28 días.
Use este medicamento inmediatamente después de su preparación. Si no se usa de inmediato, la solución preparada debe conservarse entre 2°C y 8°C y utilizarse en un plazo máximo de 8 horas. No prepare más de una dosis cada vez.
No utilice este medicamento si observa que el envase ha sido manipulado.
No utilice este medicamento si se ha conservado fuera de la nevera durante más de 28 días.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Cayston y del disolvente
- El vial de polvo contiene 75 mg de aztreonam (como lisina).
- La ampolla de disolvente contiene agua para preparaciones inyectables y cloruro de sodio. La ampolla está impresa en inglés solamente. La información que aparece en la ampolla se presenta a continuación:

Aspecto del producto y contenido del envase
Cayston es un polvo de color entre blanco y blancuzco y disolvente para solución para inhalación por nebulizador.
Cayston se encuentra en el interior de un vial de vidrio de color ámbar de 2 ml con un tapón de goma gris y un sobresellado de aluminio desprendible con una cápsula de cierre azul.
El disolvente (1 ml) se encuentra en el interior de una ampolla de plástico.
Cada envase de Cayston para 28 días contiene 84 viales de Cayston liofilizado y 88 ampollas de disolvente. Las cuatro ampollas adicionales de disolvente se suministran por si se producen derrames.
Este medicamento está disponible en:
- Envase de Cayston para 28 días
- Envase que contiene un envase de Cayston para 28 días más un dispositivo nebulizador de mano Altera
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización:
Gilead Sciences Ireland UC
Carrigtohill
County Cork, T45 DP77
Irlanda
Responsable de la fabricación:
Gilead Sciences Ireland UC
IDA Business & Technology Park
Carrigtohill
County Cork
Irlanda
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante
local del titular de la autorización de comercialización:
België/Belgique/Belgien Gilead Sciences Belgium SPRL-BVBA Tél/Tel: + 32 (0) 2 401 35 50 | Lietuva Gilead Sciences Poland Sp. z o.o. Tel: + 48 22 262 8702 |
???????? Gilead Sciences Ireland UC Te?.: + 353 (0) 1 686 1888 | Luxembourg/Luxemburg Gilead Sciences Belgium SPRL-BVBA Tél/Tel: + 32 (0) 2 401 35 79 |
Ceská republika Gilead Sciences s.r.o. Tel: + 420 910 871 986 | Magyarország Gilead Sciences Ireland UC Tel: + 353 (0) 1 686 1888 |
Danmark Gilead Sciences Sweden AB Tlf: + 46 (0) 8 5057 1849 | Malta Gilead Sciences Ireland UC Tel: + 353 (0) 1 686 1888 |
Deutschland Gilead Sciences GmbH Tel: + 49 (0) 89 899890-0 | Nederland Gilead Sciences Netherlands B.V. Tel: + 31 (0) 20 718 36 98 |
Eesti Gilead Sciences Poland Sp. z o.o. Tel: + 48 22 262 8702 | Norge Gilead Sciences Sweden AB Tlf: + 46 (0) 8 5057 1849 |
Ελλ?da Gilead Sciences Ελλ?ς Μ.ΕΠΕ. Τηλ: +30 210 8930 100 | Österreich Gilead Sciences GesmbH Tel: + 43 1 260 830 |
España Gilead Sciences, S.L. Tel: + 34 91 378 98 30 | Polska Gilead Sciences Poland Sp. z o.o. Tel: + 48 22 262 8702 |
France Gilead Sciences Tél: + 33 (0) 1 46 09 41 00 | Portugal Gilead Sciences, Lda. Tel: + 351 21 7928790 |
Hrvatska Gilead Sciences Ireland UC Tel: + 353 (0) 1 686 1888 | România Gilead Sciences Ireland UC Tel: + 353 (0) 1 686 1888 |
Ireland Gilead Sciences Ireland UC Tel: + 353 (0) 214 825 999 | Slovenija Gilead Sciences Ireland UC Tel: + 353 (0) 1 686 1888 |
Ísland Gilead Sciences Sweden ABSími: + 46 (0) 8 5057 1849 | Slovenská republika Gilead Sciences Slovakia s.r.o. Tel: + 421 232 121 210 |
Italia Gilead Sciences S.r.l. Tel: + 39 02 439201 | Suomi/Finland Gilead Sciences Sweden AB Puh/Tel: + 46 (0) 8 5057 1849 |
Κ?pρος Gilead Sciences Ελλ?ς Μ.ΕΠΕ. Τηλ: + 30 210 8930 100 | Sverige Gilead Sciences Sweden AB Tel: + 46 (0) 8 5057 1849 |
Latvija Gilead Sciences Poland Sp. z o.o. Tel: + 48 22 262 8702 | United Kingdom Gilead Sciences Ltd Tel: + 44 (0) 8000 113700 |
Fecha de la última revisión de este prospecto:
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a CAYSTON 75 mg POLVO Y DISOLVENTE PARA SOLUCION PARA INHALACION POR NEBULIZADORForma farmacéutica: INYECTABLE, 1 g aztreonamPrincipio activo: AztreonamFabricante: Galenicum Derma S.L.U.Requiere recetaForma farmacéutica: INYECTABLE PERFUSION, 1,5/0,5 gPrincipio activo: MonobactamsFabricante: Pfizer Europe Ma EeigRequiere recetaForma farmacéutica: INYECTABLE, 1 gPrincipio activo: meropenemoFabricante: Medochemie Iberia S.A.Requiere receta
Médicos online para CAYSTON 75 mg POLVO Y DISOLVENTE PARA SOLUCION PARA INHALACION POR NEBULIZADOR
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de CAYSTON 75 mg POLVO Y DISOLVENTE PARA SOLUCION PARA INHALACION POR NEBULIZADOR, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















