
BEMOLAN 2000 mg ORAL GEL

How to use BEMOLAN 2000 mg ORAL GEL
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Bemolan 2000 mg Oral Gel
Magaldrate
Read this package leaflet carefully before starting to take this medicine, as it contains important information for you.
Follow the administration instructions for the medicine contained in this package leaflet or as indicated by your doctor or pharmacist.
- Keep this package leaflet, as you may need to read it again.
- If you need advice or more information, consult your doctor or pharmacist.
- If you experience side effects, consult your doctor or pharmacist, even if they are side effects not listed in this package leaflet. See section 4.
- You should consult a doctor if your symptoms worsen or do not improve after 7 days of treatment.
Contents of the Package Leaflet:
- What Bemolan is and what it is used for.
- What you need to know before taking Bemolan.
- How to take Bemolan.
- Possible side effects.
- Storage of Bemolan.
- Contents of the pack and additional information.
1. What Bemolan is and what it is used for
Bemolan belongs to a group of medicines called antacids. The active ingredient, magaldrate, is transformed into aluminum and magnesium salts in the stomach, regulating stomach acidity.
It is indicated for the symptomatic relief of occasional gastric discomfort related to hyperacidity, stomach acid, and heartburn in adults.
2. What you need to know before taking Bemolan
Do not take Bemolan:
- if you are allergic (hypersensitive) to magaldrate or any of the other components of this medicine (listed in section 6).
- if you have intestinal obstruction.
- if you have severe kidney failure or low phosphate levels in the blood (hypophosphatemia).
- if you have high magnesium levels in the blood (hypermagnesemia).
Warnings and precautions
Consult your doctor or pharmacist before starting to take Bemolan.
Before starting treatment with Bemolan, you should inform your doctor if you have or have had any of the following disorders:
- Kidney function impairment: in very prolonged treatments and at high doses, there may be signs of chronic aluminum and/or magnesium poisoning.
- Osteoporosis (loss of bone density) and osteomalacia (softening of the bones): prolonged use and high doses of antacids containing aluminum may worsen some bone diseases due to decreased absorption of phosphorus and calcium from food.
- Dementia (progressive impairment of brain function): prolonged use and high doses of antacids containing aluminum may aggravate dementia in patients with this disease, as aluminum can accumulate in brain tissue.
Taking Bemolan with other medicines:
Inform your doctor or pharmacist if you are taking, have recently taken, or may need to take any other medicine.
You should avoid taking Bemolan simultaneously with the following medicines, as Bemolan may reduce their absorption:
- Tetracyclines and quinolone derivatives such as ciprofloxacin, ofloxacin, and norfloxacin (a group of antibiotics)
- Digoxin (used to treat heart problems)
- Benzodiazepines (used as sedatives and for sleep problems)
- Coumarin derivatives such as acenocoumarol and warfarin (oral anticoagulants)
- Indomethacin (anti-inflammatory)
- Cimetidine (antacid)
- Chenodeoxycholic and ursodeoxycholic acid (used in liver problems)
- Iron supplements (used in anemia problems)
- Isoniazid (antitubercular)
- Chlorpromazine (neuroleptic)
For this reason, the intake of the mentioned medicines should be done at least 2-3 hours before or after the administration of Bemolan.
Taking Bemolan with food and drinks:
Take this medicine 1 to 2 hours after main meals.
The concomitant use of antacids containing aluminum with acidic beverages (fruit juices, wine, etc.) may increase the intestinal absorption of aluminum. The same occurs with effervescent tablets containing citric or tartaric acid.
Pregnancy and lactation:
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Driving and using machines:
Bemolan has no or negligible influence on the ability to drive and use machines.
3. How to take Bemolan
Follow the administration instructions for the medicine contained in this package leaflet or as indicated by your doctor or pharmacist. In case of doubt, ask your doctor or pharmacist.
The recommended dose is 800 mg to 2000 mg of gel (1 sachet of 800 mg or 1 sachet of 2000 mg) orally, depending on the intensity of the symptoms, 1 to 2 hours after main meals. In certain cases, another sachet can be taken before bedtime.
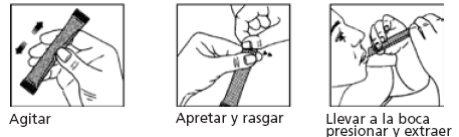
Before ingestion, it is essential to correctly remove the Bemolan sachet, pressing in different directions before opening it.
Observe the following diagram for handling the sachet:
If symptoms worsen or persist after 7 days, you should consult your doctor.
If you take more Bemolan than you should:
It is recommended not to exceed the total daily amount of 8 grams of magaldrate.
Excessive doses or usual doses in patients with a phosphate-poor diet may lead to phosphorus loss, resulting in bone and calcium loss in the urine, with a risk of osteomalacia (softening of the bones).
No cases of overdose have been reported. In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone 91 562 04 20, indicating the medicine and the amount ingested.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The side effects of Bemolan are generally mild and transient. Especially at high doses, it can cause constipation and diarrhea. However, at the recommended dose, such manifestations are very rare.
Inform your doctor as soon as possibleif you notice any of the following symptoms:
Very common side effects (may affect more than 1 in 10 patients):
- Soft stools
Very rare side effects (may affect up to 1 in 10,000 patients):
- Diarrhea and hypermagnesemia
Frequency not known (cannot be estimated from the available data):
- Neurotoxicity, encephalopathy, constipation, nausea, vomiting, abdominal pain, and hypophosphatemia.
Reporting of side effects
If you experience any type of side effect, consult your doctor, pharmacist, or nurse, even if it is a possible side effect not listed in this package leaflet. You can also report them directly through the Spanish Medicines and Health Products Agency's website: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Bemolan
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiration date stated on the packaging after CAD. The expiration date is the last day of the month indicated.
No special storage conditions are required.
Do not use Bemolan if you observe signs of deterioration of the packaging or the contents.
Medicines should not be disposed of via wastewater or household waste. Deposit the packaging and any unused medicines in the pharmacy's SIGRE point. In case of doubt, ask your pharmacist how to dispose of the packaging and any unused medicines. This will help protect the environment.
6. Contents of the pack and additional information
Composition of Bemolan
The active ingredient is: magaldrate. One 12.5 ml sachet contains 2000 mg of magaldrate.
The other ingredients are: gum arabic, hypromellose, sodium cyclamate, simethicone, methylcellulose, sorbic acid, silver sulfate, chlorhexidine digluconate (20% aqueous solution), peppermint flavor, purified water.
Appearance of the product and contents of the pack
Each sachet contains 12.5 ml of white or creamy gel with a slight mint flavor.
Presentation: 30 sachets.
Only certain pack sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder:
Takeda Farmacéutica España, S.A.
Calle Albacete, 5, 9th floor,
Edificio Los Cubos
28027 Madrid
Spain
Phone: +34 91 790 42 22
Manufacturer:
TAKEDA GMBH,
Robert Bosch Strasse, 8.
Singen, Germany
Date of the last revision of this package leaflet: December 2019
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.es/
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to BEMOLAN 2000 mg ORAL GELDosage form: ORAL SOLUTION/SUSPENSION, 800 mgActive substance: magaldrateManufacturer: Takeda Farmaceutica Espana S.A.Prescription not requiredDosage form: TABLET, 450 mgActive substance: magaldrateManufacturer: Laboratorios Ern S.A.Prescription not requiredDosage form: ORAL SOLUTION/SUSPENSION, 310 mg; 190 mg; 315 mg/5 mlActive substance: ordinary salt combinationsManufacturer: Arafarma Group S.A.Prescription not required
Online doctors for BEMOLAN 2000 mg ORAL GEL
Discuss questions about BEMOLAN 2000 mg ORAL GEL, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















