
BECLOMETHASONE/FORMOTEROL CIPLA 200 micrograms/6 micrograms/inhalation pressurized solution

How to use BECLOMETHASONE/FORMOTEROL CIPLA 200 micrograms/6 micrograms/inhalation pressurized solution
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
- Introduction
- What Beclometasone/Formoterol Cipla is and what it is used for
- What you need to know before you use Beclometasone/Formoterol Cipla
- How to use Beclometasone/Formoterol Cipla
- Possible Adverse Effects
- Conservation of Beclometasone/Formoterol Cipla
- Package Contents and Additional Information
Introduction
Leaflet: Information for the user
Beclometasone/Formoterol Cipla 200 micrograms/6 micrograms/puff, inhalation solution in a pressurised container.
beclometasone dipropionate/formoterol fumarate dihydrate
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What Beclometasone/Formoterol Cipla is and what it is used for
- What you need to know before you use Beclometasone/Formoterol Cipla
- How to use Beclometasone/Formoterol Cipla
- Possible side effects
- Storing Beclometasone/Formoterol Cipla
- Contents of the pack and other information
1. What Beclometasone/Formoterol Cipla is and what it is used for
Beclometasone/Formoterol Cipla is a pressurised inhalation solution that contains two active substances that are inhaled through the mouth and released directly into the lungs.
The two active substances are beclometasone dipropionate and formoterol fumarate dihydrate. Beclometasone dipropionate belongs to a group of medicines called corticosteroids that have an anti-inflammatory action that reduces inflammation and irritation in your lungs.
Formoterol fumarate dihydrate belongs to a group of medicines called long-acting bronchodilators that relax the muscles in the airways, making it easier for you to breathe.
These two active substances combined make breathing easier, as they provide relief from symptoms such as difficulty breathing, wheezing and coughing in asthmatic patients.
This medicine is used to treat asthma in adults.
If you have been prescribed this medicine, it is likely that:
- Your asthma is not adequately controlled by using inhaled corticosteroids and short-acting bronchodilators as needed.
or
- Your asthma responds well to a combination treatment of corticosteroids and long-acting bronchodilators.
2. What you need to know before you use Beclometasone/Formoterol Cipla
Do not use Beclometasone/Formoterol Cipla:
- If you are allergic to beclometasone dipropionate or formoterol fumarate dihydrate
or to any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor, pharmacist or nurse before starting to use Beclometasone/Formoterol Cipla if you:
- have heart problems, such as angina (heart pain, chest pain), heart failure, narrowing of the arteries, heart valve disease or any other known heart abnormality
- have high blood pressure or if you know you have an aneurysm (an abnormal bulge in the vascular wall)
- have heart rhythm disorders, such as increased heart rate or irregular heartbeat, rapid pulse or palpitations, or have been informed that you have electrocardiographic alterations
- have an overactive thyroid gland
- have low potassium levels in the blood
- have any liver or kidney disease
- have diabetes (inhaling high doses of formoterol may cause an increase in blood glucose levels and, therefore, you may need to have additional blood tests to control blood sugar levels when you start using the inhaler and from time to time during treatment).
- have a tumour of the adrenal glands (called pheochromocytoma)
- if you are going to receive anaesthesia. Depending on the type of anaesthetic, you may need to stop using this medicine at least 12 hours before anaesthesia
- if you are being or have been treated for tuberculosis (TB) or if you have a known viral or fungal lung infection
- if you must avoid drinking alcohol for any reason
If any of the above applies to you, always inform your doctor before using Beclometasone/Formoterol Cipla.
If you have or have had medical problems or allergies or if you are not sure if you can use this medicine, talk to your doctor, nurse or pharmacist before using this medicine.
Your doctor may want to measure your blood potassium levels from time to time, especially if you have severe asthma.Like many other bronchodilators, this medicine may cause a sudden drop in serum potassium levels (hypokalemia). This is because a lack of oxygen in the blood combined with other treatments you may be taking with this medicine can worsen the decrease in potassium levels.
If high doses of inhaled corticosteroids are administered for long periods, you may need more corticosteroids in stressful situations. These situations include hospitalisation after an accident, having a serious injury or before surgery. In such cases, the doctor treating you will decide if it is necessary to increase the dose of corticosteroids and may prescribe steroid tablets or injections.
In case you need to go to hospital,remember to take all your medicines and inhalers with you, including this medicine and any other medicines or tablets you have bought without a prescription, if possible in their original packaging.
Get in touch with your doctor if you experience blurred vision or other visual disturbances.
Children and adolescents
This medicine must not be used in children and adolescents under 18 years of age, until more data is available.
Other medicines and Beclometasone/Formoterol Cipla
Tell your doctor or pharmacist if you are using, have recently used or might use other medicines, including those bought without a prescription. This is because this medicine may affect the way some medicines work.
In particular, tell your doctor or pharmacist or nurse if you are using any of the following medicines:
- Some medicines may increase the effects of this medicine, so your doctor may want to monitor you closely if you are taking these medicines (including some for HIV: ritonavir, cobicistat).
- Beta-blocker medicines. Beta-blockers are medicines used to treat many conditions, such as heart problems, high blood pressure or glaucoma (increased eye pressure). If you need to use beta-blockers (including eye drops), the effect of formoterol may be reduced or even cancelled.
- Beta-adrenergic medicines (medicines with the same action as formoterol) may increase the effects of formoterol.
- Medicines to treat heart rhythm disorders (quinidine, disopyramide, procainamide).
- Medicines to treat allergic reactions (antihistamines).
- Medicines to treat depression or psychiatric disorders such as monoamine oxidase inhibitors (e.g. phenelzine, isocarboxazid) or tricyclic antidepressants (e.g. amitriptyline and imipramine), or phenothiazines.
- Medicines to treat Parkinson's disease (levodopa).
- Medicines to treat hypothyroidism (levothyroxine).
- Medicines that contain oxytocin (which causes uterine contractions).
- Medicines used to treat psychiatric disorders such as monoamine oxidase inhibitors (MAOIs), including drugs with similar properties, such as furazolidone and procarbazine.
- Medicines to treat heart conditions (digoxin).
- Other medicines used to treat asthma (theophylline, aminophylline or steroids).
- Diuretics.
Tell your doctor if you are going to have a general anaesthetic for a surgical or dental procedure.
Pregnancy, breastfeeding and fertility
There are no clinical data on the use of Beclometasone/Formoterol Cipla during pregnancy.
Do not use Beclometasone/Formoterol Cipla if you are pregnant or think you may be pregnant, if you intend to become pregnant or if you are breast-feeding, unless your doctor advises you to do so.
Driving and using machines
Beclometasone/Formoterol Cipla is unlikely to affect your ability to drive or use machines.
Beclometasone/Formoterol Cipla contains alcohol
This medicine contains 9 mg of alcohol (ethanol) in each puff. The amount of alcohol in a puff of this medicine is equivalent to less than 1 ml of beer or wine. The small amount of alcohol in this medicine does not produce any noticeable effect.
3. How to use Beclometasone/Formoterol Cipla
Beclometasone/Formoterol Cipla is for inhalation use only. This medicine should be inhaled through the mouth into the lungs.
Follow exactly the instructions for administration of this medicine given by your doctor or pharmacist. If you are in doubt, consult your doctor or pharmacist again.
Your doctor will periodically review your treatment to ensure that you are taking the optimal dose of this medicine. Your doctor will adjust the treatment to the minimum dose that best controls your symptoms.
Dose:
Adults and elderly patients:
The recommended dose is two puffs twice a day.
The maximum daily dose is 4 puffs.
Remember: you should always carry your "rescue" inhaler for rapid action with you to treat worsening asthma symptoms or a sudden asthma attack.
Patients at risk:
No dose adjustment is necessary in elderly patients. There is no information available on the use of this medicine in patients with liver or kidney problems.
Use in children and adolescents under 18 years:
Children and adolescents under 18 years of age should not take this medicine.
Beclometasone/Formoterol Cipla is effective for the treatment of asthma at a dose of beclometasone dipropionate that may be lower than that of other inhalers containing the same component. If you have previously been using another inhaler that contained beclometasone dipropionate, your doctor will advise you on the exact dose of this medicine that you should take for asthma.
Do not increase the dose.
If you think the medicine is not working very well, always consult your doctor before increasing the dose.
If your asthma gets worse
If your symptoms worsen or become difficult to control (for example, if you increase the frequency of use of the "rescue" rapid-acting inhaler), or if the "rescue" inhaler does not improve your symptoms, see your doctor immediately. Your asthma may be worsening and your doctor may need to change the dose of this medicine or prescribe an alternative treatment.
Method of administration
Beclometasone/Formoterol Cipla is for inhalation use only
This medicine is in a pressurised cartridge inside a plastic casing with a mouthpiece. There is a counter on the back of the inhaler that indicates how many doses are left. Each time you press the cartridge, a puff of medicine is released and the counter decreases by one dose. Be careful not to drop the inhaler as this could cause the counter to decrease.
Checking the inhaler
Before using the inhaler for the first time, or if you have not used it for 14 days or more, you must check your inhaler to ensure it is working correctly.
- Remove the protective cap from the mouthpiece.
- Hold the inhaler upright with the mouthpiece at the bottom.
- Point the mouthpiece away from you and press the cartridge firmly to release a puff.
- Check the dose counter. If you are checking your inhaler for the first time, the counter should indicate 120.
How to use your inhaler
When possible, stand or sit upright to perform the inhalation.
Before starting the inhalation, check the dose counter: any number between "1" and "120" shows that doses are left. If the dose counter shows "0" there are no doses left - dispose of the inhaler and get a new one.
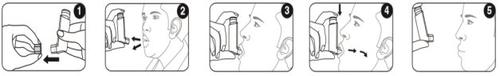
- Remove the protective cap from the mouthpiece and check that it is clean and free from dust and dirt or other foreign particles.
- Exhale slowly and deeply as much as possible.
- Hold the cartridge upright with the body upwards and place the mouthpiece between your lips. Do not bite the mouthpiece.
- Inhale slowly and deeply through your mouth and, just as you start to inhale, press the top of the inhaler firmly to release a puff. If you have weakness in your hands, you may find it easier to hold the inhaler with both hands: hold the top of the inhaler with both index fingers and the bottom with both thumbs.
- Hold your breath for as long as possible and finally remove the inhaler from your mouth and exhale slowly. Do not blow into the inhaler.
In case you need to inhale a new puff, hold the inhaler upright for about half a minute and then repeat steps 2 to 5.
Important: Do not perform steps 2 to 5 too quickly.
After administration, replace the protective cap and check the dose counter.
You should replace the inhaler when the counter shows the number 20. Stop using the inhaler when the counter shows 0, as the remaining puffs in the device may not be sufficient to take the full dose.
If you notice a "mist" escaping from the top of the inhaler or from the corner of your lips, this means that the medicine is not going to reach your lungs as it should. Take another puff following the instructions starting again from step 2.
To reduce the risk of fungal infection in the mouth and throat, rinse your mouth or gargle with water or brush your teeth each time you use the inhaler.
If you think the effect of this medicine is too strong or too weak, talk to your doctor or pharmacist.
If you find it difficult to press the inhaler while starting to inhale, you can use the AeroChamber Plus device. Talk to your doctor, pharmacist or nurse about using this device.
It is important that you read the leaflet provided with the AeroChamber Plus device and follow the instructions on how to use the AeroChamber Plus device and how to clean it carefully.
Cleaning
You should clean the inhaler once a week.
When you clean it, do not remove the cartridge from the actuator and do not use water or other liquids to clean the inhaler.
To clean the inhaler:
- Remove the mouthpiece cap from the inhaler.
- Pass a clean, dry cloth or paper towel through the inside and outside of the mouthpiece and actuator.
- Replace the mouthpiece cap.
If you use more Beclometasone/Formoterol Cipla than you should
- If you use more formoterol than you should, you may experience the following side effects: nausea, vomiting, rapid pulse, palpitations, heart rhythm disorders, certain electrocardiographic changes (heart signal), headache, tremors, drowsiness, excess acid in the blood, low potassium levels in the blood and high glucose levels in the blood. Your doctor may ask you to have blood tests to check your potassium and glucose levels in the blood.
- Taking too much beclometasone dipropionate may cause short-term changes in the functioning of the adrenal glands. This situation will improve within a few days. However, your doctor may check your cortisol levels in the blood.
Talk to your doctor if you experience any of these symptoms.
In case of overdose or accidental ingestion, talk to your doctor or pharmacist immediately or call the Toxicology Information Service, telephone 91 562 04 20, indicating the medicine and the amount taken.
If you forget to use Beclometasone/Formoterol Cipla
Do not take a double dose to make up for a forgotten dose. Take it as soon as you remember. If it is almost time for the next dose, do not take the missed dose, just take the next dose at the correct time.
If you stop using Beclometasone/Formoterol Cipla
Do not reduce the dose or stop using the medicine. Even if you feel better, do not stop using this medicine or reduce the dose. If you want to do so, consult your doctor. It is very important to use this medicine regularly, even if you do not have symptoms.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist or nurse.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them.
As with other inhaler treatments, there is a risk of worsening breathing difficulties and wheezing immediately after using beclometasone/formoterol, which is known as paradoxical bronchospasm. If this happens, SUSPEND the use of Beclometasone/Formoterol Cipla and use your rapid-acting inhaler immediatelyto treat breathing difficulty and wheezing symptoms. Contact your doctor immediately.
Inform your doctor immediately if you experience hypersensitivity reactions, such as skin allergies, itching, rash, skin redness, swelling of the skin or mucous membranes, especially in the eyes, face, lips, and throat.
Other adverse effects are listed below according to their frequency.
Frequent(may affect less than 1 in 10 people):
- fungal infections (of the mouth and throat)
- headache
- hoarseness
- sore throat
Uncommon(may affect 1 in 100 people):
- palpitations,
- exceptionally rapid heartbeat and cardiac rhythm disorders
- certain electrocardiographic alterations (ECG)
- increased blood pressure
- flu-like symptoms
- sinusitis
- rhinitis
- ear inflammation
- throat irritation
- cough and productive cough
- asthma attack
- vaginal fungal infections
- nausea
- alteration or decrease in taste
- lip burning
- dry mouth
- difficulty swallowing
- indigestion
- stomach discomfort
- diarrhea
- muscle pain and cramps
- redness of the face and throat
- increased blood flow in certain tissues of the body
- excessive sweating
- tremors
- restlessness
- dizziness
- rash or hives
- alterations in certain blood components:
- decrease in white blood cell count
- increase in platelet count
- decrease in blood potassium concentration
- increase in blood sugar
- increase in insulin, free fatty acids, and ketone bodies in blood
The following adverse effects have also been reported as “uncommon” in patients with chronic obstructive pulmonary disease:
- pneumonia; inform your doctor if you experience any of the following symptoms: increased sputum production, changes in sputum color, fever, increased cough, increased respiratory problems
- reduction in blood cortisol levels; this is caused by the effect of corticosteroids on the adrenal gland
- irregular heartbeats
Rare(may affect 1 in 1,000 people):
- chest tightness
- feeling of missed heartbeats
- decrease in blood pressure
- kidney inflammation
- persistent swelling of the skin and mucous membranes for several days
Very Rare(may affect 1 in 10,000 people):
- worsening of asthma
- breathing difficulties
- decrease in platelet count
- swelling of hands and feet
Inhalation of corticosteroids in high doses for a prolonged period may cause, in very rare cases, systemic effects,including:
- adrenal gland problems (suppression of adrenal function)
- increased intraocular pressure (glaucoma)
- cataracts
- growth retardation (slow growth in children and adolescents)
- decrease in bone mineral density (weakening of bones)
Frequency not known (cannot be estimated from available data):
- sleep problems
- depression or anxiety
- nervousness
- overexcitement or irritability
These effects may occur more frequently in children.
- blurred vision
Reporting of Adverse Effects
If you experience any type of adverse effect, consult your doctor or pharmacist or nurse, even if it is a possible adverse effect that does not appear in this prospectus. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines https://www.notificaram.es. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Conservation of Beclometasone/Formoterol Cipla
- Keep this medicine out of sight and reach of children.
- Before dispensing: store the inhaler in the refrigerator (between 2°C and 8°C)
- After dispensing (medicine dispensed by your pharmacist):
- Do not use this medicine after 3 months from the date it was dispensed by your pharmacist and never use it after the expiration date that appears on the box and label after CAD. The expiration date is the last day of the month indicated.
- Do not store the inhaler at a temperature above 25°C
- If the inhaler has been exposed to intense cold, warm it with your hands for a few minutes before use. Never heat it by artificial means.
Warning: the cartridge contains a pressurized liquid. Do not expose the cartridge to temperatures above 50°C. Do not puncture the cartridge.
Medicines should not be thrown down the drain or into the trash. Deposit the packaging and medicines you no longer need in the SIGRE collection point at the pharmacy. If in doubt, ask your pharmacist how to dispose of the packaging and medicines you no longer need. This will help protect the environment.
6. Package Contents and Additional Information
Composition of Beclometasone/Formoterol Cipla
- The active ingredients are: beclometasone dipropionate and formoterol fumarate dihydrate. Each measured dose/puff of the inhaler contains 200 micrograms of beclometasone dipropionate and 6 micrograms of formoterol fumarate dihydrate. This corresponds to a released dose from the mouthpiece of 169.2 micrograms of beclometasone dipropionate and 5.0 micrograms of formoterol.
- This medicine contains fluorinated greenhouse gases.
- Each inhaler contains 10.24 g of HFC-134a (Norflurane) which corresponds to 0.015 tons of CO2 equivalent (global warming potential GWP = 1430).
The other components are: anhydrous ethanol and hydrochloric acid.
Appearance of Beclometasone/Formoterol Cipla and Package Contents
Beclometasone/Formoterol Cipla is packaged in a 19 mL pressurized aluminum cartridge sealed with a metering valve and inserted into a plastic propylene actuator with a dose counter that incorporates a mouthpiece and is provided with a protective plastic cap.
Package sizes:
1 pressurized container that provides 120 puffs or
2 pressurized containers that provide 120 puffs each
Only some package sizes may be marketed.
Marketing Authorization Holder:
Cipla Europe NV
De Keyserlei, 60C, Bus-1301,
2018, Antwerp,
Belgium.
Manufacturer:
Cipla Europe NV
De Keyserlei, 60C, Bus-1301,
2018, Antwerp,
Belgium.
S&D Pharma CZ, spol. s.r.o.
Theodor 28, 273 08,
Pchery (Pharmos a.s. facility),
Czech Republic
You can request more information about this medicine by contacting the local representative of the marketing authorization holder:
Cipla Europe NV branch in Spain
C/Guzmán el Bueno 133, Edificio Britannia
28003, Madrid
Spain
This medicine is authorized in the Member States of the European Economic Area under the following names:
Austria: Beclometason/ Formoterol Cipla 200 Mikrogramm/6 Mikrogramm pro Sprühstoß Druckgasinhalation, Lösung
Bulgaria: BIBECFO 200/6 micrograms per actuation pressurised inhalation solution
Czech Republic: Beklometason/Formoterol Cipla
Germany: Beclometason/ Formoterol Cipla 200 Mikrogramm/6 Mikrogramm pro Sprühstoß Druckgasinhalation, Lösung
Spain: Beclometasona/Formoterol Cipla 200 micrograms/6 micrograms/puff pressurized inhalation solution
France: BÉCLOMÉTASONE/FORMOTÉROL CIPLA 200/6 microgrammes/dose, solution pour inhalation en flacon pressurisé
Italy: BIBECFO
Norway: Beklometasondipropionat/Formoterol Cipla
Romania: Beclometazona/Formoterol Cipla 200/6 micrograme pe doza solutie de inhalat presurizata
Sweden: Brofobec
Slovakia: BIBECFO 200/6 mikrogramov na inhaláciu inhalacný roztok v tlakovom obale
Date of the last revision of this prospectus:January 2025
Detailed information about this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es.
- Country of registration
- Average pharmacy price31.36 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to BECLOMETHASONE/FORMOTEROL CIPLA 200 micrograms/6 micrograms/inhalation pressurized solutionDosage form: PULMONARY INHALATION, 100 micrograms/6 micrograms/actuationActive substance: formoterol and beclometasoneManufacturer: Cipla Europe N.V.Prescription requiredDosage form: PULMONARY INHALATION, 100 micrograms/6 micrograms/actuationActive substance: formoterol and beclometasoneManufacturer: Lupin Europe GmbhPrescription requiredDosage form: PULMONARY INHALATION, 100 micrograms/6 microgramsActive substance: formoterol and beclometasoneManufacturer: Chiesi España S.A.U.Prescription required
Online doctors for BECLOMETHASONE/FORMOTEROL CIPLA 200 micrograms/6 micrograms/inhalation pressurized solution
Discuss questions about BECLOMETHASONE/FORMOTEROL CIPLA 200 micrograms/6 micrograms/inhalation pressurized solution, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















