
ATROLAK PROLONG 400MG COMPRIMIDOS DE LIBERACION PROLONGADA EFG

Cómo usar ATROLAK PROLONG 400MG COMPRIMIDOS DE LIBERACION PROLONGADA EFG
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Atrolak Prolong 400 mg comprimidos de liberación prolongada EFG
quetiapina
Lea todo el prospecto detenidamente antes de empezar a tomar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted,y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Atrolak Prolong y para qué se utiliza
- Qué necesita saber antes de empezar a tomar Atrolak Prolong
- Cómo tomar Atrolak Prolong
- Posibles efectos adversos
- Conservación de Atrolak Prolong
- Contenido del envase e información adicional
1. Qué es Atrolak Prolong y para qué se utiliza
Atrolak Prolong comprimidos de liberación prolongada contiene una sustancia denominada quetiapina. Pertenece a un grupo de medicamentos denominados antipsicóticos. Atrolak Prolong comprimidos de liberación prolongada puede utilizarse para tratar varias enfermedades, tales como:
- Esquizofrenia: por la que usted puede oír o sentir cosas que no están ahí, creer cosas que no son verdad o sentirse anormalmente receloso, ansioso, confuso, culpable, tenso o deprimido.
- Manía: por la que usted puede sentirse muy excitado, eufórico, agitado, entusiasta o hiperactivo o presentar poco juicio lo que incluye estar agresivo o violento.
- Depresión bipolar y episodios depresivos mayores en el trastorno depresivo mayor: por los que usted se siente triste. Puede encontrar que se siente deprimido, se siente culpable, con falta de energía, pérdida de apetito o que no puede dormir.
Cuando se esté utilizando Atrolak Prolong para tratar los episodios depresivos mayores en el trastorno depresivo mayor, este se tomará añadido a otro medicamento que se esté utilizando para tratar esta enfermedad.
Su médico puede continuar recetándole Atrolak Prolong comprimidos de liberación prolongada incluso cuando usted se encuentre mejor.
2. Qué necesita saber antes de empezar a tomar Atrolak Prolong
No tome Atrolak Prolong:
- Si es alérgico (hipersensible) a quetiapina o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6)
- Si está tomando alguno de los siguientes medicamentos:
- algunos medicamentos para el VIH
- medicamentos de tipo azol (para las infecciones fúngicas)
- eritromicina o claritromicina (para las infecciones)
- nefazodona (para la depresión).
No tome Atrolak Prolong comprimidos de liberación prolongada si lo anteriormente mencionado es aplicable a usted. Si tiene alguna duda, consulte a su médico o farmacéutico antes de tomar Atrolak Prolong comprimidos de liberación prolongada.
Advertencias y precauciones
Consulte a su médico o farmacéutico o enfermero antes de empezar a tomar Atrolak Prolong comprimidos de liberación prolongada
- Si padece depresión u otras afecciones que se tratan con antidepresivos. El uso de estos medicamentos junto con Quetiapina puede provocar el síndrome serotoninérgico, una afección potencialmente mortal (ver “Uso de Quetiapina con otros medicamentos”).
- Si usted, o algún familiar, tiene o ha tenido algún problema de corazón, por ejemplo, problemas en el ritmo del corazón o si está tomando cualquier medicamento que pueda afectar al latido de su corazón.
- Si tiene la tensión arterial baja.
- Si ha tenido un accidente cerebrovascular, especialmente si es paciente de edad avanzada.
- Si tiene problemas de hígado.
- Si alguna vez ha presentado un ataque epiléptico (convulsión).
- Si usted sabe que ha tenido en el pasado niveles bajos de glóbulos blancos (los cuales pueden o no haber sido causados por otros medicamentos).
- Si padece diabetes o tiene riesgo de padecer diabetes. Si es así, su médico podría controlar sus niveles de azúcar en sangre mientras esté tomando Atrolak Prolong comprimidos de liberación prolongada.
- Si usted o algún familiar tiene antecedentes de coágulos en la sangre, ya que medicamentos como éstos se han asociado con la formación de coágulos en la sangre.
- Si es una persona de edad avanzada con enfermedad de Parkinson/parkinsonismo.
- Si es una persona de edad avanzada con demencia (pérdida de funcionalidad en el cerebro). Si es así, no debe tomar Atrolak Prolong porque el grupo de medicamentos al que pertenece Atrolak Prolong puede aumentar el riesgo de accidente cerebrovascular, o en algunos casos el riesgo de fallecimiento, en estas personas.
- Si tiene o ha tenido una afección en la que su respiración se interrumpe por cortos periodos de tiempo durante el sueño nocturno normal (llamada “apnea del sueño”) y está tomando medicamentos que disminuyen la actividad normal del cerebro (“depresores”).
- Si tiene o ha tenido una afección en la que no puede vaciar completamente su vejiga (retención urinaria), tiene la próstata agrandada, una obstrucción en su intestino o presión elevada en el interior de su ojo. Estas afecciones pueden ser causadas en ocasiones por medicamentos (llamados “anti-colinérgicos”) que afectan a la forma en la que funcionan las células nerviosas, para tratar ciertas condiciones médicas.
- Si tiene antecedentes de abuso del alcohol o de las drogas.
Informe a su médico inmediatamente si experimenta algo de lo descrito a continuación después de tomar Atrolak Prolong comprimidos de liberación prolongada:
- Una combinación de fiebre, rigidez muscular intensa, sudoración o una disminución del nivel de consciencia (un trastorno denominado “síndrome neuroléptico maligno”). Puede ser necesario un tratamiento médico inmediato.
- Movimientos incontrolados, principalmente de su cara o lengua.
- Mareo o se siente muy somnoliento. Esto puede aumentar el riesgo de lesiones accidentales (caídas) en pacientes de edad avanzada.
- Ataques (crisis).
- Erección dolorosa y de larga duración (Priapismo).
Estos trastornos pueden ser causados por este tipo de medicamento.
Informe a su médico tan pronto como sea posible si usted tiene:
- Fiebre, síntomas similares a la gripe, dolor de garganta, o cualquier otra infección, ya que podría ser consecuencia de un recuento muy bajo de células blancas sanguíneas y requerir una interrupción del tratamiento con Atrolak Prolong comprimidos de liberación prolongada y/o un tratamiento adicional.
- Estreñimiento junto con dolor abdominal persistente, o estreñimiento que no ha respondido a un tratamiento, ya que podría conducir a un bloqueo más grave del intestino.
Pensamientos de suicidio y empeoramiento de su depresión
Si está deprimido, algunas veces puede pensar en hacerse daño o suicidarse. Esto puede aumentar al principio de comenzar el tratamiento, ya que todos estos medicamentos tardan tiempo en hacer efecto, por lo general alrededor de dos semanas pero algunas veces más. Estos pensamientos pueden también aumentar si deja de tomar bruscamente su medicación. Puede ser más probable que piense así si es un adulto joven. La información obtenida en los ensayos clínicos ha demostrado un aumento del riesgo de pensamientos de suicidio y/o conducta suicida en adultos jóvenes menores de 25 años con depresión.
Si en algún momento piensa en hacerse daño o suicidarse, contacte con su médico o vaya a un hospital inmediatamente. Puede servirle de ayuda decirle a un familiar o amigo cercano que está deprimido, y pedirle que lea este prospecto. Puede pedirles que le digan si ellos piensan que su depresión está empeorando, o si están preocupados por los cambios en su comportamiento.
Reacciones adversas cutáneas graves (SCARs)
Con el uso de este medicamento, se han notificado muy raramente reacciones adversas graves de la piel (SCARs), que pueden poner la vida en peligro o ser mortales. Éstas se manifiestan comunmente como:
- Síndrome de Stevens-Johnson (SSJ), una erupción generalizada con ampollas y descamación de la piel, particularmente alrededor de la boca, nariz, ojos y genitales.
- Necrólisis epidérmica tóxica (NET), una forma más grave que produce descamación extensa de la piel.
- Reacción medicamentosa con eosinofilia y síntomas sistémicos (DRESS, por sus siglas en inglés), que consiste en síntomas similares a la gripe con erupción, fiebre, gánglios inflamados y resultados anormales en el análisis de sangre (incluyendo incremento del número de glóbulos blancos (eosinofilia) y enzimas hepáticas elevadas).
- Pustulosis Exantemática Generalizada Aguda (AGEP, por sus siglas en inglés), pequeñas ampollas llenas de pus.
- Eritema multiforme (EM), erupciones en la piel con manchas rojas irregulares que pican.
Si desarrolla estos síntomas, deje de usar este medicamento y contacte con su médico o busque atención médica de inmediato.
Aumento de peso
Se ha observado aumento de peso en pacientes que toman Atrolak Prolong comprimidos de liberación prolongada. Usted y su médico deben controlar su peso regularmente.
Niños y adolescentes
Atrolak Prolong no se debe utilizar en niños y adolescentes menores de 18 años de edad.
Otros medicamentos y Atrolak Prolong comprimidos de liberación prolongada
Informe a su médico si está tomando, ha tomado recientemente o podría tener que tomar cualquier otro medicamento.
No tome Atrolak Prolong comprimidos de liberación prolongada si está utilizando alguno de los siguientes medicamentos:
- Algunos medicamentos para el VIH.
- Medicamentos de tipo azol (para las infecciones fúngicas).
- Eritromicina o claritromicina (para las infecciones).
- Nefazodona (para la depresión).
Informe a su médico si está utilizando alguno de los siguientes medicamentos:
- Medicamentos para la epilepsia (como fenitoína o carbamazepina).
- Medicamentos para la tensión arterial alta.
- Barbitúricos (para la dificultad en dormirse).
- Tioridazina (otro medicamento antipsicótico).
- Medicamentos que afecten al latido de su corazón, por ejemplo, medicamentos que pueden causar un desequilibrio en los electrolitos (niveles bajos de potasio o magnesio) tales como diuréticos (medicamentos para orinar) o ciertos antibióticos (medicamentos para tratar las infecciones).
- Antidepresivos. Estos medicamentos pueden interactuar con Quetiapina comprimidos de liberación prolongada y usted puede experimentar síntomas como contracciones rítmicas e involuntarias de los músculos, incluidos los músculos que controlan el movimiento del ojo, agitación, alucinaciones, coma, sudoración excesiva, temblor, exageración de los reflejos, aumento tensión muscular, temperatura corporal superior a 38°C (síndrome serotoninérgico). Comuníquese con su médico cuando experimente tales síntomas.
Antes de dejar de utilizar alguno de sus medicamentos, consulte primero a su médico.
Toma de Atrolak Prolong comprimidos de liberación prolongada con alimentos, bebidas y alcohol
- Atrolak Prolong comprimidos de liberación prolongada puede ser afectado por los alimentos y, por tanto, debe tomar sus comprimidos al menos una hora antes de una comida o antes de la hora de acostarse.
- Tenga cuidado con la cantidad de alcohol que ingiera. Esto es debido a que el efecto combinado de Atrolak Prolong comprimidos de liberación prolongada y alcohol puede adormecerle.
- No tome zumo de pomelo mientras esté tomando Atrolak Prolong comprimidos de liberación prolongada. Puede afectar a la forma en la que el medicamento actúa.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento. No debe tomar Atrolak Prolong comprimidos de liberación prolongada durante el embarazo, a menos que lo haya consultado a su médico. No debe utilizar Atrolak Prolong comprimidos de liberación prolongada si está en período de lactancia.
Los siguientes síntomas pueden producirse en recién nacidos, si las madres han tomado Atrolak Prolong comprimidos de liberación prolongada en el último trimestre (los tres últimos meses del embarazo): estremecimientos, rigidez y/o debilidad muscular, somnolencia, agitación, problemas respiratorios y dificultad en la alimentación. Si su bebé desarrolla algunos de estos síntomas, es posible que deba consultar con su médico.
Conducción y uso de máquinas
Estos comprimidos pueden hacer que usted se sienta adormilado. No conduzca ni maneje herramientas o máquinas hasta que usted sepa cómo le afectan los comprimidos.
Atrolak Prolong comprimidos de liberación prolongada contiene lactosa
Este medicamento contiene lactosa. Si su médico le ha indicado que padece una intolerancia a ciertos azúcares, consulte con él antes de tomar este medicamento.
Atrolak Prolong comprimidos de liberación prolongada contiene sodio
Este medicamento contiene menos de 23 mg de sodio (1 mmol) por comprimido; esto es, esencialmente “exento de sodio”.
Efecto sobre las Pruebas de Detección de Fármacos en la Orina
Si le están haciendo una prueba de detección de fármacos en la orina, la toma de Atrolak Prolong comprimidos de liberación prolongada puede producir resultados positivos para metadona o ciertos medicamentos para la depresión denominados antidepresivos tricíclicos (ATCs) cuando se utilizan algunos métodos de análisis, aunque usted pueda no estar tomando metadona ni ATCs. Si esto ocurre, se puede realizar una prueba más específica.
3. Cómo tomar Atrolak Prolong
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. Consulte a su médico o farmacéutico si tiene dudas.
La dosis recomendada (dosis diaria) dependerá de su enfermedad y de sus necesidades pero normalmente estará entre 150 mg y 800 mg.
- Tomará sus comprimidos una vez al día.
- Trague sus comprimidos enteros con ayuda de agua.
- No parta, ni mastique ni triture los comprimidos.
- Tome los comprimidos sin alimentos (al menos una hora antes de una comida o al acostarse, su médico le indicará cuándo).
- No tome zumo de pomelo mientras esté tomando Atrolak Prolong comprimidos de liberación prolongada. Puede afectar a la forma en la que el medicamento actúa.
- No deje de tomar sus comprimidos incluso si se siente mejor, a menos que su médico se lo diga.
Problemas de hígado
Si tiene problemas de hígado su médico puede cambiar su dosis.
Pacientes de edad avanzada
Si es un paciente de edad avanzada su médico puede cambiar su dosis.
Uso en niños y adolescentes
Atrolak Prolong comprimidos de liberación prolongada no debe ser utilizado por niños y adolescentes menores de 18 años de edad.
Si toma más Atrolak Prolong comprimidos de liberación prolongada de la que debiera
Si toma más Atrolak Prolong comprimidos de liberación prolongada del que le ha recetado su médico, puede sentir somnolencia, mareo y experimentar latidos cardíacos anormales. Contacte inmediatamente con su médico u hospital más próximo o llame al Servicio de Información Toxicológica, teléfono: 91 562 04 20 indicando el medicamento y la cantidad ingerida.
Si olvidó tomar Atrolak Prolong comprimidos de liberación prolongada
Si olvida tomar una dosis, tómela tan pronto como se acuerde. Si está muy próximo el momento de tomar la siguiente dosis, espere hasta entonces. No tome una dosis doble para compensarel comprimido olvidado.
Si interrumpe el tratamiento con Atrolak Prolong comprimidos de liberación prolongada
Si deja de tomar Atrolak Prolong comprimidos de liberación prolongada de forma brusca, puede ser incapaz de dormirse (insomnio), o puede sentir náuseas, o puede experimentar dolor de cabeza, diarrea, vómitos, mareo o irritabilidad. Su médico puede sugerirle reducir la dosis de forma gradual antes de interrumpir el tratamiento.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico o enfermero.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran. Si considera que alguno de los efectos adversos que sufre es grave o si aprecia cualquier efecto adverso no mencionado en este prospecto, informe a su médico o farmacéutico.
Si observa cualquiera de los siguientes efectos, debe dejar de tomar Atrolak Prolong comprimidos de liberación prolongada y contactar con su médico inmediatamente o dirigirse al hospital más cercano ya que puede necesitar atención médica urgente:
Efectos adversos muy frecuentes:pueden afectar a más de 1 de cada 10 personas
- Mareo (podría dar lugar a caídas), dolor de cabeza, sequedad de boca.
- Sensación de somnolencia (que puede desaparecer con el tiempo, a medida que siga tomando Atrolak Prolong contenidos de liberación prolongada) (podría dar lugar a caídas).
- Síntomas de discontinuación (síntomas que se producen cuando usted deja de tomar Atrolak Prolong) incluyen no ser capaz de dormir (insomnio), sentir náuseas, dolor de cabeza, diarrea, vómitos, mareo, e irritabilidad. Se aconseja una retirada gradual durante un período de al menos 1 a 2 semanas.
- Aumento de peso.
- Movimientos musculares anormales. Estos incluyen dificultad para iniciar los movimientos musculares, temblor, sensación de inquietud o rigidez muscular sin dolor.
- Cambios en la cantidad de ciertas grasas (triglicéridos y colesterol total).
Frecuentes:pueden afectar hasta 1 de cada 10 personas
- Latido cardíaco rápido.
- Sensación de que su corazón late fuertemente, muy rápido o que se salta latidos.
- Estreñimiento, estómago revuelto (indigestión).
- Sensación de debilidad.
- Edema de brazos o piernas.
- Tensión arterial baja cuando se está de pie. Esto puede hacer que usted se sienta mareado o desmayado (podría dar lugar a caídas).
- Aumento de los niveles de azúcar en la sangre.
- Visión borrosa.
- Sueños anormales y pesadillas.
- Sentirse más hambriento.
- Sentirse irritado.
- Trastorno en el habla y en el lenguaje.
- Pensamientos de suicidio y empeoramiento de su depresión.
- Falta de aliento.
- Vómitos (principalmente en pacientes de edad avanzada).
- Fiebre.
- Cambios en la cantidad de hormonas tiroideas en sangre.
- Disminución del número de ciertos tipos de células en sangre.
- Aumentos de la cantidad de enzimas hepáticas medidas en sangre.
- Aumentos de la cantidad de hormona prolactina en sangre. Los aumentos en la hormona prolactina podrían en casos raros llevar a lo siguiente:
- Tanto en hombres como en mujeres tener hinchazón de las mamas y producción inesperada de leche.
- En las mujeres no tener el período menstrual o tener períodos irregulares.
Poco frecuentes (puedenafectar a más de 1 de cada 100 personas):
- Ataques epilépticos o convulsiones.
- Reacciones alérgicas que pueden incluir ronchas abultadas (habones), hinchazón de la piel e hinchazón alrededor de la boca.
- Sensaciones desagradables en las piernas (también denominado síndrome de las piernas inquietas).
- Dificultad al tragar.
- Movimientos incontrolables, principalmente de su cara o lengua.
- Disfunción sexual.
- Diabetes
- Cambio de la actividad eléctrica del corazón vista en ECG (prolongación QT)
- Una frecuencia cardiaca más lenta de lo normal que puede ocurrir al inicio del tratamiento y que puede estar asociada con una frecuencia cardiaca lenta y desmayos
- Dificultad para orinar.
- Desmayo (podría dar lugar a caídas).
- Nariz taponada.
- Disminución en la cantidad de glóbulos rojos en sangre.
- Disminución en la cantidad de sodio en sangre.
- Empeoramiento de la diabetes pre-existente.
- Confusión.
Raros (pueden afectar hasta 1 de cada 1000 personas):
- Combinación de temperatura alta (fiebre), sudoración de larga duración, rigidez muscular, sentirse muy somnoliento o mareado (un trastorno llamado “síndrome neuroléptico maligno”).
- Color amarillento en la piel y ojos (ictericia).
- Inflamación del hígado (hepatitis)
- Erección de larga duración y dolorosa (priapismo).
- Hinchazón de las mamas y producción inesperada de leche (galactorrea).
- Trastorno menstrual.
- Coágulos de sangre en las venas especialmente en las piernas (los síntomas incluyen hinchazón, dolor y enrojecimiento en la pierna), que pueden trasladarse por los vasos sanguíneos hasta los pulmones causando dolor torácico y dificultad para respirar. Si observa alguno de estos síntomas busque inmediatamente asistencia médica.
- Sonambulismo, hablar durmiendo y desorden alimenticio relacionado con el sueño.
- Disminución de la temperatura corporal (hipotermia).
- Inflamación del páncreas (pancreatitis)
- Una condición (llamada “síndrome metabólico”) donde puede darse una combinación de 3 o más de lo siguiente: aumento de la grasa de alrededor del abdomen, descenso del “colesterol bueno” (HDL-C), aumento de un tipo de grasa en la sangre llamada triglicéridos, aumento de la presión sanguínea y aumento del azúcar en sangre.
- Una combinación de fiebre, síntomas similares a la gripe, dolor de garganta, o cualquier otra infección con un recuento de glóbulos blancos muy bajo, estado que se denomina agranulocitosis.
- Obstrucción intestinal.
- Aumento de la creatina fosfoquinasa en sangre (una sustancia de los músculos).
Muy raros (pueden afectar hasta 1 de cada 10000 personas):
- Erupción grave, ampollas, o manchas rojas en la piel.
- Una reacción alérgica severa (llamada anafilaxis) que puede causar dificultad respiratoria o shock.
- Hinchazón rápida de la piel, normalmente alrededor de los ojos, labios y garganta (angioedema).
- Una condición grave de ampollas en la piel, boca, ojos y genitales (síndrome de Stevens-Johnson). Ver sección 2.
- Secreción inapropiada de una hormona que controla el volumen de orina.
- Rotura de las fibras musculares y dolor en los músculos (rabdomiolisis).
Desconocidos: la frecuencia no puede estimarse a partir de los datos disponibles
- Erupción cutánea con manchas rojas irregulares (eritema multiforme). Ver sección 2.
- Rápida aparición de áreas de piel roja salpicadas de pequeñas pústulas (pequeñas ampollas llenas de líquido blanco/amarillo que se conocen como Pustulosis Exantemática Generalizada Aguda (AGEP). Ver sección 2.
- Reacción alérgica grave y repentina con síntomas tales como fiebre, ampollas en la piel y descamación de la piel (necrólisis epidérmica tóxica) . Ver sección 2.
- Reacción medicamentosa con eosinofilia y síntomas sistémicos (DRESS, por sus siglas en inglés), que consiste en síntomas similares a la gripe con erupción, fiebre, gánglios inflamados y resultados anormales en el análisis de sangre (incluyendo incremento del número de glóbulos blancos (eosinofilia) y enzimas hepáticas elevadas). Ver sección 2.
- Se pueden producir síntomas de abstinencia en recién nacidos de madres que hayan usado Atrolak Prolong comprimidos de liberación prolongada durante su embarazo.
- Ictus.
La clase de medicamentos a los que pertenece Atrolak Prolong comprimidos de liberación prolongada puede causar problemas en el ritmo cardíaco que pueden ser graves y en casos severos podrían ser fatales.
Algunos efectos adversos se observan solamente cuando se realiza un análisis de sangre. Estos incluyen cambios en la cantidad de ciertas grasas (triglicéridos y colesterol total) o azúcar en la sangre, cambios en la cantidad de hormonas del tiroides en sangre, aumento de las encimas hepáticas, descensos en el número de ciertos tipos de células sanguíneas, disminución en la cantidad de células rojas sanguíneas, aumento de la creatina fosfoquinasa (una sustancia de los músculos) en sangre, disminución en la cantidad de sodio en la sangre y aumentos en la cantidad de la hormona prolactina en la sangre. Los aumentos en la hormona prolactina podrían, en raros casos, dar lugar a lo siguiente:
- Tanto en hombres como en mujeres tener hinchazón de las mamas y producción inesperada de leche.
- En las mujeres no tener el período mensual o tener períodos irregulares.
Su médico puede pedirle que se haga análisis de sangre de vez en cuando.
Efectos adversos adicionales en niños y adolescentes:
Los mismos efectos adversos que pueden ocurrir en adultos pueden también ocurrir en niños y adolescentes.
Los siguientes efectos adversos se han observado más frecuentemente en niños y adolescentes:
Muy frecuentes:pueden afectar a más de 1 de cada 10 personas
- Aumento en la cantidad de una hormona denominada prolactina, en la sangre. El aumentos en la hormona prolactina podría, en raros casos, dar lugar a lo siguiente:
- Tanto en niños como en niñas tener hinchazón de las mamas y producción inesperada de leche.
- En las niñas no tener el período mensual o tener períodos irregulares.
- Aumento del apetito.
- Vómitos
- Movimientos musculares anormales. Estos incluyen dificultad al empezar los movimientos musculares, temblores, sensación de inquietud o rigidez muscular sin dolor.
Frecuentes: pueden afectar hasta 1 de cada 10 personas
- Sensación de debilidad, desmayo (podría dar lugar a caídas)
- Nariz taponada
- Sentirse irritado
Notificación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de medicamentos de Uso Humano: https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Atrolak Prolong
- Mantener fuera de la vista y del alcance de los niños.
- No utilice este medicamento después de la fecha de caducidad que aparece en la etiqueta, estuche y blister después de CAD. La fecha de caducidad es el último día del mes que se indica.
- No utilice este medicamento si observa indicios visibles de deterioro.
- Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma ayudará a proteger el medio ambiente.
Este medicamento no requiere condiciones especiales de conservación.
6. Contenido del envase e información adicional
Composición de Atrolak Prolong comprimidos de liberación prolongada
- El principio activo es quetiapina. Cada comprimido de Atrolak Prolong comprimidos de liberación prolongada contiene 400 mg de quetiapina (como fumarato de quetiapina).
- Los demás componentes son:
Núcleo del comprimido: lactosa monohidrato, hidroxipropilmetilcelulosa (hipromelosa), cloruro de sodio, povidona K-30, talco, estearato de magnesio.
Recubrimiento del comprimido: dióxido de titanio (E171), macrogol (E1521) e hidroxipropilmetilcelulosa (hipromelosa 6cP) (E464).
Aspecto de Atrolak Prolong comprimidos de liberación prolongada y contenido del envase
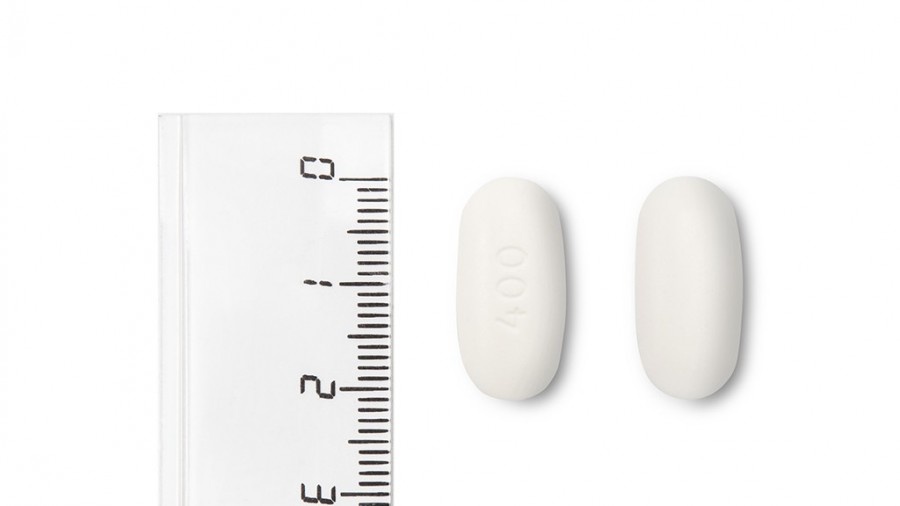
Atrolak Prolong comprimidos de liberación prolongada 400 mg son comprimidos blancos, redondos, biconvexos, recubiertos con película, grabados con la inscripción “I4” en una de las caras y lisos por la otra cara. Los comprimidos tienen un diámetro de aproximadamente 12,8 mm.
Envases blíster PVC/PVDC-Aluminio. Están registrados tamaños de envases de 10, 30, 50, 60 y 100 comprimidos.
Puede que solamente estén comercializados algunos tamaños de envase.
Titular de la autorización de comercialización
Accord Healthcare S.L.U.
World Trade Center
Moll de Barcelona, s/n
Edifici Est, 6ª planta
08039 Barcelona
España
Responsable de la fabricación
GA PHARMACEUTICALS GAP
46, Agisilaou St., Agios Dimitiros
17341 – Attica
Grecia
O
Accord Healthcare Polska Sp.z o.o.,
ul. Lutomierska 50,95-200 Pabianice,
Polonia
O
Accord Healthcare B.V.,
Winthontlaan 200,
3526 KV Utrecht
Países Bajos
O
Accord Healthcare Single Member S.A.
64th Km National Road Athens
Lamia, 32009
Grecia
Este medicamento está autorizado en los Estados Miembros del Espacio Económico Europeo y del Reino Unido (Irlanda del Norte) con los siguientes nombres:
Nombre del Estado Miembro | Nombre del medicamento | |
AT | Quetiapine Accord 400 mg Retardtabletten | |
BG | Quetiapine Accord 400mg Prolonged-release Tablets | |
CY | Quetiapine Accord 400mg Prolonged-release Tablets | |
CZ | Quetiapine Accord 400mg tablety s prodlouženým uvolnováním | |
DE | Quetiapine Accord 400mg Retardtabletten | |
DK | Quetiapine Accord Healthcare 400mg Depottabletter | |
EE | Quetiapine Accord | |
EL | Quetiapine Accord 400mg παρατε?νει δισκ?ο ελεγχ?μενης αποδ?σμευσης | |
ES | Atrolak Prolong 400mg comprimidos de liberación prolongada | |
FI | Quetiapine Accord 400mg depottabletit | |
HU | Quetiapine Accord 400mg retard tabletta | |
IE | Notiabolfen XL 400 mg prolonged-release Tablet | |
IT | Quetiapina Accord 400mg compresse a rilascio prolungato | |
LV | Quetiapine Accord 400mg ap ilgstošas darbibas tabletes | |
LT | Quetiapine Accord 400mg pailginto atpalaidavimo tabletes | |
MT | Atrolak XL 400 mg prolonged-release Tablet | |
NL | Quetiapine Accord 400mg tabletten met verlengde afgifte | |
NO | Quetiapine Accord | |
PL | KETREL XR | |
PT | Quetiapina Accord 400mg comprimidos de libertação prolongada | |
RO | Quetiapina Accord 400 mg comprimate cu eliberare prelungita | |
SE | Quetiapine Accord 400mg depottabletter | |
SI | Kvetiapin Accord 400mg tablete s podaljšanim sprošcanjem | |
SK | Quetiapine Accord 400mg Filmom obalené tablety s predlženým uvolnovaním | |
UK (Irlanda del Norte) | Atrolak XL 400 mg prolonged-release Tablet |
Fecha de última revisión de este prospecto: Julio 2024
La información detallada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/.
- País de registro
- Precio medio en farmacia129.63 EUR
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a ATROLAK PROLONG 400MG COMPRIMIDOS DE LIBERACION PROLONGADA EFGForma farmacéutica: INHALACIÓN PULMONAR, 9,1 MGPrincipio activo: LoxapinaFabricante: Ferrer Internacional S.A.Requiere recetaForma farmacéutica: INHALACIÓN PULMONAR, 9,1 mgPrincipio activo: LoxapinaFabricante: Ferrer Internacional S.A.Requiere recetaForma farmacéutica: COMPRIMIDO, 100 mgPrincipio activo: clozapinaFabricante: Aristo Pharma GmbhRequiere receta
Médicos online para ATROLAK PROLONG 400MG COMPRIMIDOS DE LIBERACION PROLONGADA EFG
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de ATROLAK PROLONG 400MG COMPRIMIDOS DE LIBERACION PROLONGADA EFG, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















