
AIDRAELLE DIARIO 3 mg/0.02 mg FILM-COATED TABLETS

How to use AIDRAELLE DIARIO 3 mg/0.02 mg FILM-COATED TABLETS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
aidraelle Daily 3 mg/0.02 mg film-coated tablets EFG
drospirenone/ethinylestradiol
Read the entire package leaflet carefully before starting to take this medication, as it contains important information for you.Because it contains important information for you.
- Keep this package leaflet, as you may need to read it again.
- If you have any questions, consult your doctor or pharmacist.
- This medication has been prescribed to you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, consult your doctor or pharmacist, even if they are side effects not listed in this package leaflet. See section 4.
Important things you should know about combined hormonal contraceptives (CHCs):
- They are one of the most reliable reversible contraceptive methods if used correctly.
- They slightly increase the risk of blood clots in veins and arteries, especially in the first year or when restarting the use of a combined hormonal contraceptive after a pause of 4 weeks or more.
- Be alert and consult your doctor if you think you may have symptoms of a blood clot (see section 2 "Blood clots").
Contents of the package leaflet
- What is aidraelle Daily and what is it used for
- What you need to know before taking aidraelle Daily
- Do not take aidraelle Daily
- Warnings and precautions
- Blood clots
- aidraelle Daily and cancer
- Intermenstrual bleeding
- What should you do if you do not have your menstrual period during the placebo days?
- Other medications and aidraelle Daily
- Taking aidraelle Daily with food and drinks
- Laboratory tests
- Pregnancy and lactation
- Driving and using machines
- aidraelle Daily contains lactose
- How to take aidraelle Daily
- When can you start with the first blister pack?
- If you take more aidraelle Daily than you should
- If you forget to take aidraelle Daily
- What should you do in case of vomiting or severe diarrhea?
- Menstrual period delay: what should you know?
- Change of the first day of your menstrual period: what should you know?
- If you interrupt treatment with aidraelle Daily
- Possible side effects
- Storage of aidraelle Daily
- Package contents and additional information
1. What is aidraelle Daily and what is it used for
aidraelle Daily is a contraceptive and is used to prevent pregnancy.
Each of the 21 active pink film-coated tablets contains a small amount of two different female hormones, called drospirenone and ethinylestradiol.
The 7 white film-coated tablets do not contain active ingredients and are called placebo tablets.
Contraceptives that contain two hormones are called combined contraceptives.
2. What you need to know before you start taking aidraelle Daily
General considerations
Before you start taking this medication, you should read the information about blood clots in section 2. It is particularly important that you read the symptoms of a blood clot (see section 2 "Blood clots").
Before you start taking this medication, your doctor will ask you some questions about your personal and family medical history. The doctor will also measure your blood pressure and, depending on your state of health, may perform other tests.
This prospectus describes several situations in which you should interrupt the use of drospirenone/ethinylestradiol, or in which its effect may decrease.
In such situations, you should not have sexual intercourse or should take additional non-hormonal contraceptive precautions, such as using a condom or another barrier method. Do not use the rhythm method or the temperature method. These methods may not be reliable since drospirenone/ethinylestradiol alters the monthly changes in body temperature and cervical mucus.
aidraelle Daily, like other hormonal contraceptives, does not protect against HIV infection (AIDS) or any other sexually transmitted disease.
Do not takeaidraelle Daily
You should not take drospirenone/ethinylestradiol if you have any of the conditions listed below. Inform your doctor if you have any of the conditions listed below. Your doctor will discuss with you what other form of contraception would be more suitable.
- If you have (or have ever had) a blood clot in a blood vessel in your legs (deep vein thrombosis, DVT), in your lungs (pulmonary embolism, PE), or in other organs.
- If you know you have a disorder that affects blood clotting: for example, protein C deficiency, protein S deficiency, antithrombin III deficiency, factor V Leiden, or antiphospholipid antibodies.
- If you need an operation or if you spend a lot of time without getting up (see section "Blood clots").
- If you have ever had a heart attack or a stroke.
- If you have (or have ever had) angina pectoris (a condition that causes severe chest pain and may be the first sign of a heart attack) or a transient ischemic attack (TIA, temporary symptoms of a stroke).
- If you have any of the following diseases that may increase your risk of forming a blood clot in the arteries:
- Severe diabetes with blood vessel damage.
- Very high blood pressure.
- Very high levels of fat in the blood (cholesterol or triglycerides).
- A condition called hyperhomocysteinemia.
- If you have (or have ever had) a type of migraine called "migraine with aura".
- If you have (or have ever had) a liver disease and your liver function has not yet normalized.
- If your kidneys do not work well (renal failure).
- If you have (or have ever had) a tumor in the liver.
- If you have (or have ever had), or if you suspect you have breast cancer or cancer of the sex organs.
- If you have vaginal bleeding, whose cause is unknown.
- If you are allergic to ethinylestradiol or drospirenone, or to any of the other components of this medication (included in section 6). This may manifest with itching, rash, or inflammation.
Do not take aidraelle Daily if you have hepatitis C and are taking medications that contain ombitasvir/paritaprevir/ritonavir and dasabuvir, glecaprevir/pibrentasvir, or sofosbuvir/velpatasvir/voxilaprevir (see also section "Other medications and aidraelle Daily").
Additional information on special populations
Children and adolescents
Drospirenone/ethinylestradiol is not indicated for use in women who have not yet had their first menstrual period.
Older women
Drospirenone/ethinylestradiol is not indicated for use after menopause.
Women with liver insufficiency
Do not take drospirenone/ethinylestradiol if you suffer from liver disease. See sections "Do not take aidraelle Daily" and "Warnings and precautions".
Women with renal insufficiency
Do not take drospirenone/ethinylestradiol if you are suffering from kidney malfunction or acute renal failure. See sections "Do not take aidraelle Daily" and "Warnings and precautions".
Warnings and precautions
Consult your doctor or pharmacist before starting to take this medication.
When should you consult your doctor? Seek urgent medical attention
To obtain a description of the symptoms of these serious side effects, see "How to recognize a blood clot". |
Tell your doctor if you suffer from any of the following conditions.
In some situations, you will need to be particularly careful while taking this medication or any other combined contraceptive, and it may be necessary for your doctor to examine you periodically.
If the condition develops or worsens while you are taking this medication, you should also inform your doctor.
- If a close relative has or has ever had breast cancer.
- If you have any liver or gallbladder disease.
- If you have diabetes.
- If you have depression.
- If you have Crohn's disease or ulcerative colitis (chronic inflammatory bowel disease).
- If you have hemolytic uremic syndrome (HUS, a blood clotting disorder that causes kidney failure).
- If you have sickle cell anemia (a hereditary disease of red blood cells).
- If you have high levels of fat in the blood (hypertriglyceridemia) or a known family history of this condition. Hypertriglyceridemia has been associated with an increased risk of pancreatitis (inflammation of the pancreas).
- If you need an operation or spend a lot of time without getting up (see section 2 "Blood clots").
- If you have just given birth, you are at a higher risk of blood clots. You should ask your doctor when you can start taking aidraelle Daily after childbirth.
- If you have inflammation of the veins that are under the skin (superficial thrombophlebitis).
- If you have varicose veins.
- If you have epilepsy (see section "Other medications and aidraelle Daily").
- If you have systemic lupus erythematosus (SLE, a disease that affects your natural defense system).
- If you have a disease that may have appeared for the first time during pregnancy or during previous use of sex hormones (e.g., hearing loss, a blood disease called porphyria, blistering skin rash during pregnancy (gestational herpes), a nervous disease in which involuntary movements appear (Sydenham's chorea)). If you have or have ever had chloasma (brownish patches, also known as "pregnancy patches", especially on the face and neck). In this case, you should avoid direct exposure to the sun or ultraviolet rays.
- If you experience symptoms such as swelling of the face, tongue, and/or throat, and/or difficulty swallowing or urticaria, with possible difficulty breathing, contact a doctor immediately. Products containing estrogens can cause or worsen the symptoms of hereditary and acquired angioedema.
BLOOD CLOTS
The use of a combined hormonal contraceptive like aidraelle Daily increases your risk of having a blood clotcompared to not using it. In rare cases, a blood clot can block blood vessels and cause serious problems.
Blood clots can form:
- In the veins (this is called "venous thrombosis", "venous thromboembolism", or VTE).
- In the arteries (this is called "arterial thrombosis", "arterial thromboembolism", or ATE).
Recovery from blood clots is not always complete. In rare cases, there can be serious long-term effects or, very rarely, they can be fatal.
It is important to remember that the overall risk of a harmful blood clot due toaidraelle Dailyis small.
HOW TO RECOGNIZE A BLOOD CLOT
Seek urgent medical attention if you notice any of the following signs or symptoms.
Are you experiencing any of these signs? | What might you be suffering from? |
| Deep vein thrombosis |
If you are unsure, consult a doctor, as some of these symptoms, such as cough or shortness of breath, can be confused with a milder condition such as a respiratory infection (e.g., a "common cold"). | Pulmonary embolism |
Symptoms that occur more frequently in one eye:
| Retinal vein thrombosis (blood clot in the eye). |
| Heart attack. |
Sometimes the symptoms of a stroke can be brief, with almost immediate and complete recovery, but you should still seek urgent medical attention, as you may be at risk of having another stroke. | Stroke |
| Blood clots that block other blood vessels. |
BLOOD CLOTS IN A VEIN
What can happen if a blood clot forms in a vein?
- The use of combined hormonal contraceptives has been associated with an increased risk of blood clots in the veins (venous thrombosis). However, these side effects are rare. They occur more frequently in the first year of use of a combined hormonal contraceptive.
- If a blood clot forms in a vein in the leg or foot, it can cause deep vein thrombosis (DVT).
- If a blood clot moves from the leg and lodges in the lung, it can cause a pulmonary embolism.
- In very rare cases, a blood clot can form in a vein in another organ, such as the eye (retinal vein thrombosis).
When is the risk of a blood clot in a vein higher?
The risk of a blood clot in a vein is higher during the first year in which you take a combined hormonal contraceptive for the first time. The risk may also be higher if you start taking a combined hormonal contraceptive (the same medication or a different one) after an interruption of 4 weeks or more.
After the first year, the risk decreases, but it is always slightly higher than if you were not taking a combined hormonal contraceptive.
When you stop taking this medication, your risk of a blood clot returns to normal within a few weeks.
What is the risk of a blood clot?
The risk depends on your natural risk of VTE and the type of combined hormonal contraceptive you are taking.
The overall risk of a blood clot in the leg or lung (DVT or PE) with aidraelle Daily is small.
- Out of 10,000 women who do not use a combined hormonal contraceptive and are not pregnant, about 2 will have a blood clot in a year.
- Out of 10,000 women who use a combined hormonal contraceptive that contains levonorgestrel, norethisterone, or norgestimate, about 5-7 will have a blood clot in a year.
- Out of 10,000 women who use a combined hormonal contraceptive that contains drospirenone, like this medication, between 9 and 12 women will have a blood clot in a year.
- The risk of a blood clot depends on your personal history (see "Factors that increase your risk of a blood clot" below).
Risk of a blood clot in a year | |
Women who do not usea combined hormonal contraceptive and are not pregnant | About 2 out of 10,000 women |
Women who use a combined hormonal contraceptive that contains levonorgestrel, norethisterone, or norgestimate | About 5-7 out of 10,000 women |
Women who use this medication | About 9-12 out of 10,000 women |
Factors that increase your risk of a blood clot in a vein
The risk of a blood clot with this medication is small, but some conditions increase the risk. Your risk is higher:
- If you are overweight (body mass index or BMI over 30 kg/m2).
- If any of your close relatives have had a blood clot in the leg, lung, or other organ at a young age (i.e., before the age of 50). In this case, you may have a hereditary blood clotting disorder.
- If you need an operation or spend a lot of time without getting up due to an injury or illness, or if you have a leg in a cast. You may need to stop using this medication several weeks before the operation or while you have reduced mobility. If you need to stop using this medication, consult your doctor about when you can start using it again.
- As you get older (especially above 35 years).
- If you have recently given birth.
The risk of a blood clot increases with the number of conditions you have.
Long-distance air travel (more than 4 hours) may temporarily increase the risk of a blood clot, especially if you have any of the other risk factors listed.
It is important to inform your doctor if you suffer from any of the above conditions, even if you are not sure. Your doctor may decide that you should stop using this medication.
If any of the above conditions change while you are using this medication, for example, a close relative experiences a blood clot without a known cause, or you gain a lot of weight, inform your doctor.
BLOOD CLOTS IN AN ARTERY
What can happen if a blood clot forms in an artery?
Like a blood clot in a vein, a blood clot in an artery can cause serious problems. For example, it can cause a heart attack or a stroke.
Factors that increase your risk of a blood clot in an artery
It is important to note that the risk of a heart attack or stroke due to the use of this medication is very small, but it may increase:
- With age (above 35 years).
- If you smoke.
- When using a combined hormonal contraceptive like aidraelle Daily, you are advised to stop smoking. If you are unable to stop smoking and are over 35 years old, your doctor may advise you to use a different type of contraceptive.
- If you are overweight.
- If you have high blood pressure.
- If any of your close relatives have had a heart attack or stroke at a young age (less than 50 years). In this case, you may also be at higher risk of having a heart attack or stroke.
- If you or any of your close relatives have high levels of fat in the blood (cholesterol or triglycerides).
- If you have migraines, especially migraines with aura.
- If you have a heart condition (valve disorder, heart rhythm disorder called atrial fibrillation).
- If you have diabetes.
If you have one or more of these conditions, or if any of them are particularly severe, the risk of a blood clot may be further increased.
If any of the above conditions change while you are using aidraelle Daily, for example, you start smoking, a close relative experiences a blood clot without a known cause, or you gain a lot of weight, inform your doctor.
aidraelle Dailyand cancer
Women who use combined contraceptives have a slightly higher rate of breast cancer, but it is not known if this is due to the treatment. For example, it may be that more tumors are detected in women who take combined contraceptives because they are examined by a doctor more frequently. The incidence of breast tumors decreases gradually after stopping combined hormonal contraceptives. It is essential to undergo regular breast examinations, and you should see your doctor if you notice any lump.
In rare cases, benign liver tumors, and even more rarely, malignant liver tumors, have been reported in users of hormonal contraceptives. See your doctor if you have severe abdominal pain...
3. How to take aidraelle Daily
Follow the instructions for administration of this medication indicated by your doctor or pharmacist. In case of doubt, consult your doctor or pharmacist again.
Each blister pack contains 21 film-coated active pink tablets and 7 film-coated white placebo tablets.
The two different types of colored tablets of aidraelle Daily are arranged in order. A blister pack contains 28 tablets.
Take one aidraelle Daily tablet each day with a glass of water if necessary. You can take the tablets with or without food, but approximately at the same time every day.
Do not get confused with the tablets: take one pink tablet every day for the first 21 days, and then one white tablet for the last 7 days. Then, you should start a new blister pack (21 pink tablets and 7 white tablets). This way, there is no week of rest between two blister packs.
Due to the different composition of the tablets, it is necessary to start with the first tablet located in the upper left area and then take one tablet each day. To maintain the order, follow the direction of the arrows on the blister pack.
Preparing the blister pack
To help you follow the order of intake, each aidraelle Daily blister pack contains 7 self-adhesive strips with the 7 days of the week. Choose the strip of the week that starts with the day you take the first tablet. For example, if you take your first tablet on a Wednesday, use the strip that starts with “WED”.
Stick the self-adhesive strip of the week on the top of the aidraelle Daily blister pack, where it says “Place the strip that matches the start date here”, so that the first day is placed above the tablet marked with “Start”.
This way, there is a day of the week indicated above each tablet, and you can see if you have taken a particular tablet. The arrows show the order in which you should take the tablets.
During the 7 days when you take the white placebo tablets (the placebo days), you usually start your period (also called withdrawal bleeding). Usually, the period starts on the second or third day after taking the last active pink tablet of aidraelle Daily. Once you have taken the last white tablet, you should start the next blister pack, even if you have not finished your period. This means that you should start each blister pack on the same day of the week that you started the previous one, and that your menstruation should take place during the same days each month.
If you take aidraelle Daily as indicated, you are also protected against pregnancy during the 7 days when you are taking the placebo tablets.
When can you start with the first blister pack?
If you have not taken any hormonal contraceptive in the previous month.
Start taking this medication on the first day of your cycle (i.e., the first day of your menstruation).
If you start aidraelle Daily on the first day of your menstruation, you will be immediately protected against pregnancy.
You can also start on days 2-5 of your cycle, but you must use additional contraceptive methods (e.g., a condom) during the first 7 days.
Changing from another combined hormonal contraceptive, vaginal ring, or patch.
You can start taking this medication preferably the day after taking the last active tablet (the last tablet that contains active ingredients) of your previous contraceptive, or at the latest the day after the rest week of your previous contraceptive (or after taking the last inactive tablet of your previous contraceptive). When changing from a combined vaginal ring or patch, follow your doctor's recommendations.
Changing from a progestin-only method (progestin-only pill, injection, implant, or intrauterine device).
You can change from the progestin-only pill at any time. If it is an implant or an intrauterine device, on the day of its removal; if it is an injectable, when the next injection is due. In all cases, it is recommended that you use additional contraceptive measures (e.g., a condom) during the first 7 days of tablet-taking.
After an abortion
Follow your doctor's recommendations.
After having a child.
You can start taking this medication between 21 and 28 days after giving birth. If you start later, you must use a barrier method (e.g., a condom) during the first 7 days of using this medication.
If, after having a child, you have had sexual intercourse before starting to take this medication again, you must first be sure that you are not pregnant or wait for your next menstrual period.
If you are breastfeeding and want to start takingdrospirenone/ethinylestradiolagain after having a child.
See section “Pregnancy and Breastfeeding”.
Ask your doctor if you are unsure when to start.
If you take moreaidraelle Dailythan you should
No cases have been reported where an overdose with this medication has caused serious harm.
The symptoms that may appear if you take many tablets at once may include feeling unwell or vomiting or vaginal bleeding. This bleeding can occur even in girls who have not yet had their first menstrual period, if they accidentally take this medication.
If you have taken too many tablets of this medication, or discover that a child has taken them, consult your doctor or pharmacist.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone 91 562 04 20, indicating the medication and the amount ingested.
If you forget to takeaidraelle Daily
The tablets in the fourthrow of the blister pack are the placebo tablets. If you forget to take one of these tablets, it will have no effect on the reliability of aidraelle Daily. Discard the forgotten placebo tablet.
If you forget to take an active pink tablet from the 1st, 2nd, or 3rdrow, do the following:
- If you are less than 12 hourslate in taking a tablet, the protection against pregnancy is not reduced. Take the tablet as soon as you remember and the following tablets at the usual time.
- If you are more than 12 hourslate in taking a tablet, the protection against pregnancy may be reduced. The more tablets you have forgotten, the higher the risk of becoming pregnant.
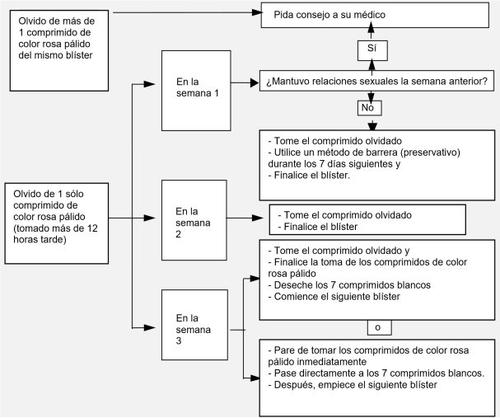
The risk of incomplete protection against pregnancy is highest if you forget to take a pink tablet at the beginning of the blister pack (1st row) or at the end of the blister pack (3rd row of the blister pack). Therefore, you should follow the following recommendations (see the diagram below).
Forgetting more than one tablet in the blister pack
Consult your doctor.
Forgetting a tablet in week 1
Take the forgotten tablet as soon as you remember, even if it means taking two tablets at the same time. Continue taking the tablets at the usual time and use additional precautions, such as a condom, for the next 7 days. If you have had sexual intercourse in the week before forgetting the tablet, you should know that there is a risk of pregnancy. In this case, consult your doctor.
Forgetting a tablet in week 2
Take the forgotten tablet as soon as you remember, even if it means taking two tablets at the same time. Continue taking the tablets at the usual time. The protection against pregnancy is not reduced, and you do not need to take additional precautions.
Forgetting a tablet in week 3
You can choose between two options:
- Take the forgotten tablet as soon as you remember, even if it means taking two tablets at the same time. Continue taking the tablets at the usual time. Instead of taking the white placebo tablets from this blister pack, discard them and start taking the next blister pack.
- You will probably have your period at the end of the second blister pack, while taking the white placebo tablets, although you may experience light bleeding or spotting during the second blister pack. You can also stop taking the active pink tablets and go directly to the 7 white placebo tablets (before taking the placebo tablets, you must note the day you forgot to take the tablet). If you want to start a new blister pack on the day you always start, take the placebo tablets for less than 7 days.
If you follow one of these two recommendations, you will remain protected against pregnancy. If you have forgotten to take a tablet and do not have a period during the placebo days, you may be pregnant. In this case, you should go to your doctor before continuing with the next blister pack.
What should you do in case of vomiting or severe diarrhea?
If you vomit within 3-4 hours after taking an active pink tablet or suffer from severe diarrhea, there is a risk that the active ingredients of the contraceptive will not be fully absorbed by the body. The situation is almost equivalent to forgetting a tablet. After vomiting or diarrhea, you should take a pink tablet from a reserve blister pack as soon as possible. If possible, take it within 12 hoursafter the usual time you take your contraceptive. If it is not possible or more than 12 hours have passed, follow the advice in the section “If you forget to take aidraelle Daily”.
Delayed menstrual period: what should you know?
Although it is not recommended, it is possible to delay your menstrual period if you do not take the white placebo tablets from the fourth row and start taking a new blister pack of drospirenone/ethinylestradiol and finish it. You may experience light bleeding or spotting during the use of the second blister pack. Finish this second blister pack by taking the 7 white tablets from the 4th row. Then, start with the next blister pack.
You must consult your doctor before deciding to delay your menstrual period.
Changing the first day of your period: what should you know?
If you take the tablets as instructed, your menstrual period will start during the week of taking placebo tablets. If you need to change that day, reduce the number of days of taking white placebo tablets (but never increase them – 7 at most!). For example, if you normally start taking the placebo tablets on Friday and want to change it to Tuesday (3 days earlier), you should start a new blister pack 3 days earlier than usual. If you make the placebo period very short (e.g., 3 days or less), you may not experience bleeding during these days. Then, you may experience light bleeding or spotting.
If you are unsure how to proceed, consult your doctor.
If you stop treatment with aidraelle Daily
You can stop taking this medication whenever you want. If you do not want to become pregnant, consult your doctor about other effective birth control methods.
If you want to become pregnant, stop taking this medication and wait until your menstrual period before trying to become pregnant. This way, you can more easily calculate the estimated date of delivery.
If you have any other questions about the use of this medication, ask your doctor or pharmacist.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them. If you experience any adverse effect, especially if it is severe and persistent, or have any change in health that you think may be due to this medicine, consult your doctor.
Contact a doctor immediately if you experience any of the following symptoms of angioedema: swelling of the face, tongue and/or throat, and/or difficulty swallowing or urticaria with possible difficulty breathing (see also section "Warnings and Precautions").
All women who take combined hormonal contraceptives are at a higher risk of developing blood clots in the veins (venous thromboembolism (VTE)) or blood clots in the arteries (arterial thromboembolism (ATE)). For more detailed information on the different risks of taking combined hormonal contraceptives, see section 2 "What you need to know before taking aidraelle Daily".
The following list of adverse effects has been associated with the use of this medicine.
Frequent Adverse Effects(may affect up to 1 in 10 people)
- mood changes.
- headache.
- abdominal pain (stomach pain).
- acne.
- breast pain, breast enlargement, breast tension, painful or irregular menstrual periods.
- weight gain.
Uncommon Adverse Effects(may affect up to 1 in 100 people)
- candidiasis (a fungal infection).
- cold sore (herpes simplex).
- allergic reactions.
- increased appetite.
- depression, nervousness, sleep disorders.
- tingling and pinching, dizziness.
- vision problems.
- irregular or unusually fast heart rate.
- blood clots (thrombosis) in the lungs (pulmonary embolism), increased blood pressure, decreased blood pressure, migraine, varicose veins.
- sore throat.
- nausea, vomiting, inflammation of the stomach and/or intestine, diarrhea, constipation.
- sudden swelling of the skin and/or mucous membranes (e.g., tongue or throat), and/or difficulty swallowing or urticaria with breathing difficulties (angioedema), hair loss (alopecia), eczema, itching, skin rashes, dry skin, seborrheic dermatitis.
- neck pain, limb pain, muscle cramps.
- bladder infection.
- breast lumps (benign or cancerous), milk production without being pregnant (galactorrhea), ovarian cysts, hot flashes, absence of menstruation, heavy menstrual bleeding, vaginal discharge, vaginal dryness, pain in the lower abdominal region (pelvic), abnormal cervical smears (Pap smear or Pap staining), decreased libido.
- fluid retention, lack of energy, excessive thirst, increased sweating.
- weight loss.
Rare Adverse Effects(may affect up to 1 in 1,000 people):
- asthma.
- hearing problems.
- erythema nodosum (characterized by painful red nodules on the skin).
- erythema multiforme (skin rash with target-shaped redness or ulcers).
- harmful blood clots in a vein or artery, for example:
- in a leg or foot (i.e., DVT).
- in a lung (i.e., PE).
- heart attack.
- stroke.
- mild or temporary stroke-like symptoms, known as a transient ischemic attack (TIA).
- blood clots in the liver, stomach/intestine, kidneys, or eye.
The likelihood of having a blood clot may be higher if you have any other condition that increases this risk (see section 2 for more information on conditions that increase the risk of blood clots and symptoms of a blood clot).
Reporting Adverse Effects
If you experience any type of adverse effect, consult your doctor or pharmacist, even if it is a possible adverse effect that is not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines https://www.notificaram.es. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of aidraelle Daily
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiration date stated on the packaging after CAD. The expiration date is the last day of the month indicated.
Do not store above 30°C.
Medicines should not be disposed of via wastewater or household waste. Deposit the packaging and any unused medicines in the pharmacy's SIGRE Point. In case of doubt, ask your pharmacist how to dispose of the packaging and any unused medicines. This will help protect the environment.
6. Package Contents and Additional Information
Composition ofaidraelle Daily
- The active ingredients are drospirenone and ethinylestradiol. Each active pink film-coated tablet contains 3 milligrams of drospirenone and 0.02 milligrams of ethinylestradiol.
- The white film-coated tablets do not contain active ingredients.
- The other components are:
Active pink film-coated tablets:
Tablet core: lactose monohydrate, potassium polacrylate, povidone, magnesium stearate.
Coating: Opadry II pink, which contains: macrogol, poly(vinyl alcohol), titanium dioxide (E-171), talc (E-553b), yellow iron oxide (E-172), and red iron oxide (E-172).
Inactive white film-coated tablets:
Tablet core: lactose monohydrate, potassium polacrylate, povidone, anhydrous colloidal silica, magnesium stearate.
Coating: Opadry II white, which contains: macrogol, poly(vinyl alcohol), titanium dioxide (E-171), and talc (E-553b).
Appearance and Package Contents
Each aidraelle Daily blister pack contains 21 active pink film-coated tablets in the 1st, 2nd, and 3rd rows of the blister pack and 7 inactive white tablets in the 4th row.
aidraelle Daily is available in cardboard boxes of 1 and 3 blister packs, each containing a PVC/PVDC/Aluminum blister pack with 28 film-coated tablets.
The active aidraelle Daily tablets are cylindrical, biconvex, pink, and approximately 6 mm in diameter.
The placebo tablets are cylindrical, biconvex, white, and approximately 6 mm in diameter.
Each cardboard box contains a sleeve to store the blister pack.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder
Laboratorios Cinfa, S.A.
Carretera Olaz-Chipi, 10. Polígono Industrial Areta
31620 Huarte (Navarra) - Spain
Manufacturer
Cyndea Pharma, S.L
Pol. Ind. Emiliano Revilla Sanz
Avenida de Ágreda, 31
42110 Ólvega (Soria) Spain
Date of the Last Revision of this Leaflet:November 2024
Detailed information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/.
You can access detailed and updated information on this medicine by scanning the QR code included in the leaflet and packaging with your smartphone. You can also access this information at the following internet address: https://cima.aemps.es/cima/dochtml/p/78878/P_78878.html
QR code to: https://cima.aemps.es/cima/dochtml/p/78878/P_78878.html
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to AIDRAELLE DIARIO 3 mg/0.02 mg FILM-COATED TABLETSDosage form: TABLET, 3 mg/0.03 mgActive substance: drospirenone and ethinylestradiolManufacturer: Laboratorios Cinfa S.A.Prescription requiredDosage form: TABLET, 3 mg/0.03 mgActive substance: drospirenone and ethinylestradiolManufacturer: Laboratorios Cinfa S.A.Prescription requiredDosage form: TABLET, 3 mg/0.02 mgActive substance: drospirenone and ethinylestradiolManufacturer: Laboratorios Cinfa S.A.Prescription required
Online doctors for AIDRAELLE DIARIO 3 mg/0.02 mg FILM-COATED TABLETS
Discuss questions about AIDRAELLE DIARIO 3 mg/0.02 mg FILM-COATED TABLETS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions






