
Como usar XELJANZ 1 mg/mL SOLUÇÃO ORAL
Introdução
Prospecto: informação para o paciente
XELJANZ 1 mg/ml solução oral
tofacitinibe
Leia todo o prospecto detenidamente antes de começar a tomar este medicamento, porque contém informações importantes para si.
- Conserva este prospecto, porque pode ter que voltar a lê-lo.
- Se tiver alguma dúvida, consulte o seu médico ou farmacêutico.
- Este medicamento foi prescrito apenas para si, e não deve dá-lo a outras pessoas, mesmo que tenham os mesmos sintomas que si, porque pode prejudicá-las.
- Se experimentar efeitos adversos, consulte o seu médico ou farmacêutico, mesmo que se trate de efeitos adversos que não aparecem neste prospecto. Ver seção 4.
Além deste prospecto, o seu médico também lhe dará um cartão de informação para o paciente, que contém informações importantes de segurança que precisa conhecer antes de tomar XELJANZ e durante o tratamento com XELJANZ. Mantenha este cartão de informação para o paciente consigo.
Conteúdo do prospecto
- O que é XELJANZ e para que é utilizado
- O que precisa saber antes de começar a tomar XELJANZ
- Como tomar XELJANZ
- Posíveis efeitos adversos
- Conservação de XELJANZ
- Conteúdo do envase e informação adicional
- Instruções para o uso de XELJANZ solução oral
1. O que é XELJANZ e para que é utilizado
XELJANZ 1 mg/ml solução oral é um medicamento que contém o princípio ativo tofacitinibe.
XELJANZ 1 mg/ml solução oral é utilizado para o tratamento da artrite idiopática juvenil poliarticular ativa, uma doença de longa duração que principalmente produz dor e inflamação das articulações em pacientes com 2 anos de idade ou mais.
XELJANZ 1 mg/ml solução oral também é utilizado para o tratamento da artrite psoriásica juvenil, uma afecção que é uma doença inflamatória das articulações, muitas vezes acompanhada de psoríase, em pacientes com 2 anos de idade ou mais.
XELJANZ 1 mg/ml solução oral pode ser utilizado em combinação com metotrexato quando o tratamento prévio para a artrite idiopática juvenil poliarticular ou para a artrite psoriásica juvenil não foi eficaz ou não foi bem tolerado. XELJANZ 1 mg/ml solução oral também pode ser tomado como único medicamento nos casos em que o tratamento com metotrexato não é tolerado ou não é recomendado.
2. O que precisa saber antes de começar a tomar XELJANZ
Não tome XELJANZ
- se é alérgico ao tofacitinibe ou a algum dos outros componentes deste medicamento
- (incluídos na seção 6)
- se tem uma infecção grave como infecção do sangue ou tuberculose ativa
- se lhe foi informado que tem problemas de fígado graves, como cirrose (cicatrizes no fígado)
- se está grávida ou em período de amamentação
Por favor, contacte o seu médico se tiver dúvidas sobre algum dos pontos anteriores.
Advertências e precauções
Consulte o seu médico ou farmacêutico antes de começar a tomar XELJANZ:
- se acredita que tem uma infecção ou tem sintomas de uma infecção como febre, suor, calafrios, dores musculares, tosse, dificuldade para respirar, aparecimento de catarro ou alterações no catarro, perda de peso, pele quente, vermelha ou dolorosa ou feridas no corpo, dificuldade ou dor ao engolir, diarreia ou dor de estômago, ardor ao urinar ou urina com mais frequência do que o normal, ou se sente muito cansado
- se padece alguma doença que aumente a probabilidade de infecção (por exemplo, diabetes, VIH/SIDA ou um sistema imunológico débil)
- se padece algum tipo de infecção, está a receber tratamento para alguma infecção, ou se padece infecções que voltam a aparecer. Informe o seu médico imediatamente se não se sente bem. XELJANZ pode reduzir a capacidade do corpo para responder às infecções e pode agravar uma infecção existente ou aumentar a probabilidade de contrair uma nova infecção
- se padece ou tem antecedentes de tuberculose ou esteve em contacto próximo com alguém com tuberculose. O seu médico realizará um teste de tuberculose antes de começar o tratamento com XELJANZ e pode voltar a realizar o teste durante o tratamento
- se padece alguma doença pulmonar crónica
- se tem problemas no fígado
- se padece ou já padeceu hepatite B ou hepatite C (vírus que afetam o fígado). O vírus pode ativar-se enquanto estiver a tomar XELJANZ. O seu médico pode realizar análises de sangue para a hepatite antes de começar o tratamento com XELJANZ e enquanto estiver a tomar XELJANZ
- se já padeceu algum tipo de cancro, e também se fuma atualmente ou já fumou no passado. XELJANZ pode aumentar o risco de certos tipos de cancro. Foram notificados casos de cancro de leucócitos, cancro de pulmão e outros tipos de cancro (como cancro de mama, pele, próstata e pâncreas) em pacientes tratados com XELJANZ. Se desenvolver cancro enquanto estiver em tratamento com XELJANZ, o seu médico avaliará se deve interromper o tratamento com XELJANZ
- se tem um risco conhecido de fracturas, por exemplo, se tem mais de 65 anos, é mulher ou toma corticosteroides (por exemplo, prednisona)
- se tem um risco alto de desenvolver cancro de pele, o seu médico pode recomendar que faça revisões de pele periódicas enquanto estiver a tomar XELJANZ
- se já padeceu diverticulite (um tipo de inflamação do intestino grosso) ou úlceras no estômago ou nos intestinos (ver seção 4)
- se tem problemas renais
- se tem intenção de se vacinar, informe o seu médico. Não se devem administrar certos tipos de vacinas quando se toma XELJANZ. Antes de começar a tomar XELJANZ, deve estar atualizado com todas as vacinas recomendadas. O seu médico decidirá se precisa ser vacinado contra o herpes zóster
- se padece problemas cardíacos, pressão arterial alta, colesterol alto, e também se fuma atualmente ou já fumou no passado.
Foram notificados casos de pacientes tratados com XELJANZ que desenvolveram coágulos de sangue nos pulmões ou nas veias. O seu médico reverá o seu risco de desenvolver coágulos sanguíneos nos pulmões ou nas veias e determinará se XELJANZ é apropriado para si. Se já teve problemas por desenvolver coágulos sanguíneos nos pulmões e nas veias ou tem um maior risco de desenvolvê-los [por exemplo, se tem sobrepeso importante, cancro, problemas cardíacos, diabetes, já teve um ataque cardíaco (nos 3 meses anteriores), já teve uma cirurgia maior recente, se utiliza anticoncepcionais hormonais/terapia hormonal substitutiva, se lhe foi identificada alguma anomalia na coagulação a si ou a familiares próximos], se fuma atualmente ou já fumou no passado, o seu médico pode decidir que XELJANZ não é apropriado para si.
Consulte o seu médico imediatamente se apresentar falta de ar ou dificuldade para respirar de forma repentina, dor no peito ou dor na parte superior das costas, inchaço nas pernas ou nos braços, dor ou sensibilidade à palpação nas pernas, ou vermelhidão ou alteração de cor nas pernas ou braços enquanto toma XELJANZ, porque estes podem ser sinais de um coágulo nos pulmões ou nas veias.
Consulte o seu médico imediatamente se experimenta alterações graves na visão (visão borrada, perda parcial ou total de visão), porque isto pode ser um sinal de coágulos sanguíneos nos olhos.
Foram notificados casos de pacientes tratados com XELJANZ que tiveram um problema cardíaco, incluindo um infarto do miocárdio. O seu médico avaliará o seu risco para desenvolver um problema cardíaco e determinará se XELJANZ é apropriado para si. Fale com o seu médico imediatamente se apresentar sinais e sintomas de infarto do miocárdio, como dor torácica grave ou opressão (que pode estender-se aos braços, mandíbula, pescoço, costas), dificuldade para respirar, suor frio, tontura ou tonturas repentinas.
Testes adicionais de controlo
O seu médico deve realizar análises de sangue antes de começar a tomar XELJANZ, após 4 a 8 semanas de tratamento e luego cada 3 meses, para determinar se tem um recuento baixo de glóbulos brancos (neutrófilos ou linfócitos) ou um recuento baixo de glóbulos vermelhos (anemia).
Não deve tomar XELJANZ se o seu recuento de glóbulos brancos (neutrófilos ou linfócitos) ou o seu recuento de glóbulos vermelhos for demasiado baixo. Se for necessário, o seu médico pode suspender o tratamento com XELJANZ para reduzir o risco de infecção (recuento de glóbulos brancos) ou anemia (recuento de glóbulos vermelhos).
O seu médico também pode realizar outros testes, por exemplo, para controlar os níveis de colesterol no sangue ou vigiar o estado do seu fígado. O seu médico deve avaliar os seus níveis de colesterol nas 8 semanas seguintes ao início do tratamento com XELJANZ. O seu médico deve realizar testes hepáticos periodicamente.
Pacientes de idade avançada
Não foi estabelecida a segurança e eficácia de tofacitinibe 1 mg/ml solução oral em pacientes de idade avançada.
Pacientes asiáticos
Observa-se um maior número de herpes zóster em pacientes japoneses e coreanos. Informe o seu médico se notar bolhas dolorosas na pele.
Também pode ter um maior risco de padecer determinados problemas pulmonares. Informe o seu médico se notar alguma dificuldade para respirar.
Crianças e adolescentes
Este medicamento não deve ser administrado a pacientes menores de 2 anos de idade.
Este medicamento contém propilenoglicol e deve ser utilizado com precaução em pacientes de 2 anos de idade ou mais e apenas se foi recomendado pelo médico (ver "XELJANZ contém propilenoglicol").
Outros medicamentos e XELJANZ
Informe o seu médico ou farmacêutico se está a tomar, tomou recentemente ou pode ter que tomar qualquer outro medicamento.
Informe o seu médico se tem diabetes ou está a tomar medicamentos para tratar a diabetes. O seu médico pode decidir que precisa de menos medicamento antidiabético enquanto toma tofacitinibe.
Alguns medicamentos não devem ser tomados com XELJANZ. Se forem tomados com XELJANZ, podem alterar o nível de XELJANZ no seu corpo, e a dose de XELJANZ pode requerer um ajuste. Informe o seu médico se está a utilizar medicamentos que contenham algum dos seguintes princípios ativos:
- antibióticos como rifampicina, utilizados para tratar infecções bacterianas
- fluconazol, cetoconazol, utilizados para tratar infecções fúngicas
Não se recomenda o uso de XELJANZ com medicamentos que deprimam o sistema imunológico, incluindo as denominadas terapias biológicas dirigidas (anticorpos), tais como aqueles que inibem o factor de necrose tumoral, a interleucina-17, a interleucina-12/interleucina-23, os antagonistas das integrinas e fortes imunossupressores químicos, incluindo azatioprina, mercaptopurina, ciclosporina e tacrolimo. O uso de XELJANZ com estes medicamentos pode aumentar o risco de efeitos adversos, incluindo infecção.
Podem aparecer infecções graves e fracturas com mais frequência em pessoas que também tomam corticosteroides (por exemplo, prednisona).
Gravidez e amamentação
Se é uma mulher em idade fértil, deve usar anticoncepcionais eficazes durante o tratamento com XELJANZ e durante pelo menos 4 semanas após a última dose.
Se está grávida ou em período de amamentação, acredita que pode estar grávida ou tem intenção de engravidar, consulte o seu médico antes de utilizar este medicamento. Não se deve usar XELJANZ durante a gravidez. Informe o seu médico imediatamente se engravidar enquanto toma XELJANZ.
Se está a tomar XELJANZ e em período de amamentação, deixe de dar o peito até que fale com o seu médico sobre a interrupção do tratamento com XELJANZ.
Condução e uso de máquinas
XELJANZ não tem ou tem um efeito limitado sobre a sua capacidade para conduzir ou utilizar máquinas.
XELJANZ contém propilenoglicol
Este medicamento contém 2,39 mg de propilenoglicol em cada ml de solução oral.
XELJANZ contém benzoato de sódio
Este medicamento contém 0,9 mg de benzoato de sódio em cada ml de solução oral.
XELJANZ contém sódio
Este medicamento contém menos de 1 mmol de sódio (23 mg) por ml; isto é, essencialmente “isento de sódio”.
3. Como tomar XELJANZ
Este medicamento foi facultado e supervisionado por um médico especialista que sabe como tratar a sua doença.
Siga exatamente as instruções de administração deste medicamento indicadas pelo seu médico, não se deve exceder a dose recomendada. Em caso de dúvida, consulte novamente o seu médico ou farmacêutico.
A dose recomendada em pacientes a partir dos 2 anos de idade baseia-se na seguinte classificação segundo o peso (ver Tabela 1):
Tabela 1: Dose de XELJANZ para pacientes com artrite idiopática juvenil poliarticular e APs juvenil desde os dois anos de idade:
Peso corporal (kg) | Esquema de dosificação |
10 - < 20 | 3,2 mg (3,2 ml de solução oral) duas vezes ao dia |
20 - < 40 | 4 mg (4 ml de solução oral) duas vezes ao dia |
≥ 40 | 5 mg (5 ml de solução oral ou 5 mg comprimidos revestidos com película) duas vezes ao dia |
O seu médico pode reduzir a dose se tiver problemas de fígado ou rim, ou se lhe for prescrito determinados medicamentos. O seu médico também pode interromper o tratamento de forma temporária ou permanente se os análises de sangue mostram recuentos baixos de glóbulos brancos ou glóbulos vermelhos.
Se tem artrite idiopática juvenil poliarticular ou artrite psoriásica juvenil, o seu médico pode mudar o seu tratamento de XELJANZ 5 ml solução oral duas vezes ao dia para XELJANZ 5 mg comprimidos revestidos com película duas vezes ao dia.
XELJANZ é para uso por via oral. Pode tomar XELJANZ com ou sem alimentos.
Tente tomar XELJANZ à mesma hora todos os dias (pela manhã e à noite).
Se tomar mais XELJANZ do que deve
Se tomar mais XELJANZ 1 mg/ml solução oral do que deve, informe imediatamente o seu médico ou farmacêutico.
Se esquecer de tomar XELJANZ
Não tome uma dose dupla para compensar as doses esquecidas. Tome a próxima dose à hora habitual e continue como antes.
Se interromper o tratamento com XELJANZ
Não deixe de tomar XELJANZ sem consultar o seu médico.
Se tiver alguma outra dúvida sobre o uso deste medicamento, pergunte ao seu médico ou farmacêutico.
4. Possíveis efeitos adversos
Tal como todos os medicamentos, este medicamento pode produzir efeitos adversos, embora nem todas as pessoas os sofram.
Alguns podem ser graves e necessitar atenção médica.
Os efeitos adversos em pacientes com artrite idiopática juvenil poliarticular e artrite psoriásica juvenil foram consistentes com os observados em pacientes adultos com artrite reumatoide, com a exceção de algumas infecções (gripe, faringite, sinusite, infecção viral) e distúrbios gastrointestinais ou gerais (dor abdominal, náuseas, vómitos, febre, dor de cabeça, tosse), que foram mais frequentes na população pediátrica com artrite idiopática juvenil.
Possíveis efeitos adversos graves
Em casos raros, as infecções podem ser mortais. Também se notificaram casos de cancro do pulmão, cancro dos glóbulos brancos e de enfarte do miocárdio.
Se notar algum dos seguintes efeitos adversos graves, informe o seu médico imediatamente.
Os sinais de infecção grave (frequentes) incluem
- febre e calafrios
- tosse
- bolhas na pele
- dor de estômago
- dor de cabeça persistente
Os sinais de úlceras ou furos (perfurações) no estômago (pouco frequentes) incluem
- febre
- dor no estômago ou dor abdominal
- sangue nas fezes
- mudanças não justificadas nos hábitos intestinais
As úlceras no estômago ou intestino ocorrem com maior frequência nos pacientes que também estão em tratamento com medicamentos anti-inflamatórios não esteroideos ou corticosteroides (por exemplo, prednisona).
Os sinais de reações alérgicas (frequência não conhecida) incluem
- opressão no peito
- sibilância
- tontura grave ou sensação de vertigem
- inchaço dos lábios, língua ou garganta
- urticária (prurido e habões)
Os sinais de coágulos sanguíneos nos pulmões, nas veias ou nos olhos (pouco frequentes: tromboembolismo venoso) incluem
- falta de ar ou dificuldade para respirar de forma repentina
- dor no peito ou dor na parte superior das costas
- inchaço das pernas ou dos braços
- dor ou sensibilidade à palpação nas pernas
- vermelhidão ou mudança de cor das pernas ou braços
- mudanças graves na visão
Os sinais de enfarte do miocárdio (pouco frequentes) incluem
- dor ou opressão no peito (que podem estender-se aos braços, queixo, pescoço e costas)
- dificuldade em respirar
- sudorese fria
- atordoamento ou tontura repentina
Outros efeitos adversosque se observaram com XELJANZ são enumerados a seguir.
Frequentes(podem afetar até 1 em cada 10 pacientes): infecções pulmonares (pneumonia e bronquite), herpes zóster, infecções nas fossas nasais, garganta ou traqueia (nasofaringite), gripe, sinusite, infecção da bexiga urinária (cistite), dor de garganta (faringite), aumento de enzimas musculares no sangue (sinais de problemas nos músculos), dor de estômago (tripa) (que pode dever-se à inflamação do revestimento do estômago), vómitos, diarreia, mal-estar (náuseas), dispepsia, baixa contagem de glóbulos brancos, baixa contagem de glóbulos vermelhos (anemia), inchaço dos pés e mãos, dor de cabeça, pressão arterial alta (hipertensão), tosse, erupção cutânea.
Pouco frequentes(podem afetar até 1 em cada 100 pacientes): cancro do pulmão, tuberculose, infecção renal, infecção da pele, herpes simples ou úlceras bucais (herpes labial), aumento da creatinina no sangue (um possível sinal de problemas nos rins), aumento do colesterol (incluindo aumento de LDL), febre, fadiga (cansaço), aumento de peso, desidratação, desgarro muscular, tendinite, inchaço das articulações, entorse das articulações, sensações anormais, sono insuficiente, congestão sinusal, falta de ar ou dificuldade para respirar, vermelhidão da pele, prurido, fígado gordo, inflamação dolorosa das pequenas bolsas que sobressaem do revestimento interno do intestino (diverticulite), infecções virais, infecções virais que afetam o intestino, alguns tipos de cancro da pele (do tipo não melanoma).
Raros(podem afetar até 1 em cada 1 000 pacientes): infecção do sangue (sepsis), linfoma (cancro dos glóbulos brancos), tuberculose disseminada que afeta ossos e outros órgãos, outras infecções incomuns, infecção das articulações, aumento das enzimas do fígado no sangue (sinal de problemas no fígado), dor nos músculos e articulações.
Muito raros(podem afetar até 1 em cada 10 000 pacientes): tuberculose que afeta o cérebro e a medula espinhal, meningite, infecção dos tecidos moles e da fáscia.
Em geral, na artrite reumatoide se observaram menos efeitos adversos quando XELJANZ se administrava sozinho do que em combinação com metotrexato.
Comunicação de efeitos adversos
Se experimentar algum tipo de efeito adverso, consulte o seu médico ou farmacêutico, mesmo que se trate de possíveis efeitos adversos que não aparecem neste folheto. Também pode comunicá-los diretamente através do sistema nacional de notificação incluído no Apêndice V. Mediante a comunicação de efeitos adversos, você pode contribuir para fornecer mais informações sobre a segurança deste medicamento.
5. Conservação de XELJANZ
Mantenha este medicamento fora da vista e do alcance das crianças.
Não utilize este medicamento após a data de validade que aparece no cartucho ou no frasco. A data de validade é o último dia do mês que se indica.
Este medicamento não requer nenhuma temperatura especial de conservação.
Conservar no frasco e no embalagem original para protegê-lo da luz.
Elimine após 60 dias desde a primeira abertura
Não utilize este medicamento se observar que a solução mostra sinais visíveis de deterioração.
Os medicamentos não devem ser jogados nos esgotos nem na lixeira. Pergunte ao seu farmacêutico como se livrar dos envoltórios e dos medicamentos que já não precisa. Desta forma, ajudará a proteger o meio ambiente.
6. Conteúdo do envoltório e informações adicionais
Composição de XELJANZ
- O princípio ativo é tofacitinibe.
- Cada ml contém 1 mg de tofacitinibe (como citrato de tofacitinibe).
- Os demais componentes são aroma de uva [que contém propilenoglicol (E1520) (ver seção 2 “XELJANZ contém propilenoglicol”), glicerina (E422) e aromas naturais], ácido clorídrico, ácido láctico (E270), água purificada, benzoato de sódio (E211) (ver seção 2 “XELJANZ contém benzoato de sódio” e “XELJANZ contém sódio”), sucralose (E955) e xilitol (E967).
Aspecto do produto e conteúdo do envoltório
XELJANZ 1 mg/ml solução oral é uma solução transparente, incolor.
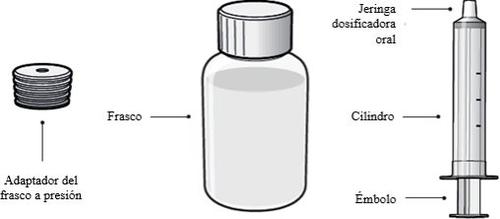
A solução oral 1 mg/ml é apresentada em frascos de HDPE de 250 ml de cor branca que contêm 240 ml de solução oral. Cada envoltório contém um frasco de HDPE, um adaptador do frasco a pressão e uma seringa dosificadora oral com as graduações de 3,2 ml, 4 ml e 5 ml.
Título da autorização de comercialização
Pfizer Europe MA EEIG
Boulevard de la Plaine 17
1050 Bruxelas
Bélgica
Responsável pela fabricação
Pfizer Service Company BVBA
Hoge Wei 10
1930 Zaventem
Bélgica
Podem solicitar mais informações sobre este medicamento dirigindo-se ao representante local do título da autorização de comercialização:
Bélgica / Bélgica / Bélgica Luxemburgo / Luxemburgo Bélgica / Bélgica / Bélgica Pfizer S.A. / N.V. Tel: +32 (0)2 554 62 11 Luxemburgo / Luxemburgo Pfizer S.A. Tel: +32 (0)2 554 62 11 | Lituânia Pfizer Luxembourg SARL filial em Lituânia Tel: +3705 2514000 |
| Hungria Pfizer Kft. Tel: +36 1 488 37 00 |
República Checa Pfizer, spol. s r.o. Tel: +420 283 004 111 | Malta Vivian Corporation Ltd. Tel: +35621 344610 |
Dinamarca Pfizer ApS Tlf: +45 44 20 11 00 | Países Baixos Pfizer bv Tel: +31 (0)10 406 43 01 |
Alemanha Pfizer Pharma GmbH Tel: +49 (0)30 550055-51000 | Noruega Pfizer AS Tlf: +47 67 52 61 00 |
Estônia Pfizer Luxembourg SARL filial na Estônia Tel: +372 666 7500 | Áustria Pfizer Corporation Austria Ges.m.b.H. Tel: +43 (0)1 521 15-0 |
Grécia PFIZER ΕΛΛΑΣ A.E. Τηλ.: +30 210 67 85 800 | Polônia Pfizer Polska Sp. z o.o., Tel.: +48 22 335 61 00 |
Espanha Pfizer, S.L. Tel: +34 91 490 99 00 | Portugal Laboratórios Pfizer, Lda. Tel: +351 21 423 5500 |
França Pfizer Tél: +33 (0)1 58 07 34 40 | Romênia Pfizer Romênia S.R.L. Tel: +40 21 207 28 00 |
Croácia Pfizer Croácia d.o.o. Tel: +385 1 3908 777 | Eslovênia Pfizer Luxembourg SARL Pfizer, filial para consultoria em farmacêutica Tel.: +386 (0) 1 52 11 400 |
Irlanda Pfizer Healthcare Irlanda Tel: 1800 633 363 (gratuito) +44 (0)1304 616161 | República Eslovaca Pfizer Luxembourg SARL, filial Tel: +421-2-3355 5500 |
Islândia Icepharma hf. Sími: +354 540 8000 | Finlândia Pfizer Oy Puh/Tel: +358 (0)9 430 040 |
Itália Pfizer S.r.l. Tel: +39 06 33 18 21 | Suécia Pfizer AB Tel: +46 (0)8 550 520 00 |
Chipre PFIZER ΕΛΛΑΣ Α.Ε. (sucursal do Chipre) Τηλ: +357 22 817690 | Reino Unido (Irlanda do Norte) Pfizer Limited Tel: +44 (0)1304 616161 |
Letônia Pfizer Luxembourg SARL filial na Letônia Tel.: +371 670 35 775 |
Data da última revisão deste folheto:
Outras fontes de informação
A informação detalhada deste medicamento está disponível no site da Agência Europeia de Medicamentos: http://www.ema.europa.eu.
- Instruções para o uso de XELJANZ solução oral
Leia estas Instruções de Uso antes de começar a tomar XELJANZ solução oral. É possível que haja nova informação.
Informação importante sobre a dosagem de XELJANZ solução oral
Use sempre a seringa dosificadora oral que se fornece com XELJANZ solução oral para medir e administrar a dosagem prescrita. Se não tiver certeza, solicite ajuda ao profissional de saúde ou ao farmacêutico para que o mostre como medir a dosagem prescrita.
Como se deve conservar XELJANZ?
Mantenha este medicamento fora da vista e do alcance das crianças.
Elimine a solução oral de XELJANZ restante uma vez transcorridos 60 dias.
Para ajudá-lo a lembrar quando deve eliminar o frasco de XELJANZ, pode escrever a data do primeiro uso no envoltório e, em seguida:
Data do primeiro uso ____/____/____.
Antes de cada uso:
Lave as mãos com água e sabão e coloque o conteúdo da caixa sobre uma superfície limpa e plana.
Cada envoltório de XELJANZ solução oral contém
- 1 adaptador do frasco a pressão
- 1 frasco de XELJANZ solução oral
- 1 seringa dosificadora oral
Passo 1. Retire o frasco do envoltório
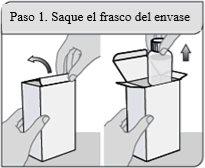
Retire o frasco de XELJANZ solução oral do envoltório.
Passo 2. Abra o frasco
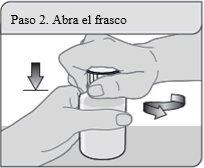
Abra o frasco. Retire o fecho selado da parte superior do frasco (somente na primeira vez).
Não jogue a tampa à prova de crianças.
Nota:Não é necessário agitar o frasco antes de usá-lo.
Passo 3. Insira o adaptador do frasco a pressão
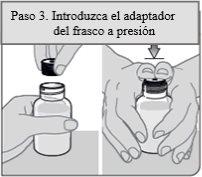
Retire o adaptador do frasco a pressão e a seringa dosificadora oral do envoltório de plástico. Com o frasco sobre uma superfície plana, empurre o extremo acanalado do adaptador do frasco a pressão com os polegares até o fundo do pescoço do frasco enquanto segura o frasco firmemente.
Nota:Não retire o adaptador do frasco a pressão após tê-lo inserido.
Passo 4. Retire o ar da seringa dosificadora oral
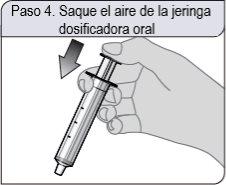
Empurre completamente o êmbolo da seringa dosificadora oral em direção ao extremo do cilindro da seringa para eliminar o excesso de ar.
Passo 5. Insira a seringa dosificadora oral
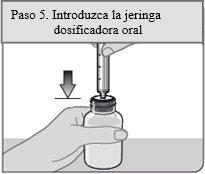
Insira a seringa dosificadora oral de forma vertical no frasco através da abertura do adaptador do frasco a pressão até que esteja firmemente colocada no lugar.
Passo 6. Retire a dosagem do frasco
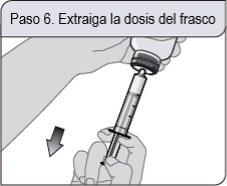
Com a seringa dosificadora oral no lugar, coloque o frasco boca abaixo. Puxe o êmbolo para trás.
Se observar bolhas de ar na seringa dosificadora oral, pressione completamente o êmbolo para devolver a solução oral ao frasco. Posteriormente, retire a dosagem de solução oral prescrita.
Passo 7. Retire a seringa dosificadora oral
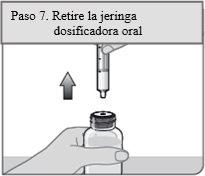
Coloque o frasco em posição vertical e coloque-o sobre uma superfície plana. Retire a seringa dosificadora oral do adaptador do frasco puxando o cilindro da seringa dosificadora oral para cima.
Passo 8. Verifique a dosagem retirada
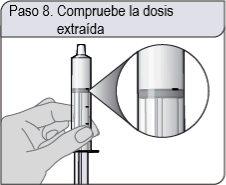
Verifique se retirou a dosagem correta na seringa dosificadora oral.
Se a dosagem não for a correta, insira firmemente o extremo da seringa dosificadora oral no adaptador do frasco. Empurre completamente o êmbolo para que a solução oral retorne ao frasco. Repita os passos 6 e 7.
Passo 9. Tome a dosagem de XELJANZ
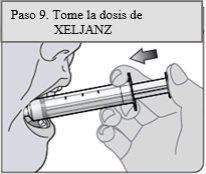
Coloque a ponta da seringa dosificadora oral no interior da bochecha do paciente.
Empurre lentamente o êmbolo até o fundo para administrar todo o medicamento da seringa dosificadora oral. Certifique-se de que o paciente tenha tempo para engolir o medicamento.
Passo 10. Feche o frasco
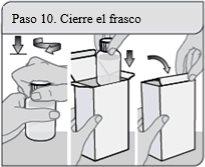
Feche bem o frasco girando a tampa à prova de crianças no sentido dos ponteiros do relógio, deixe o adaptador do frasco a pressão onde está.
Volte a colocar o frasco no envoltório e feche-o para proteger a solução oral de XELJANZ da luz.
Passo 11. Limpe a seringa dosificadora oral
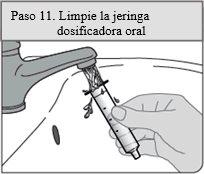
Retire o êmbolo do cilindro da seringa puxando o êmbolo e o cilindro em direções diferentes.
Enxágue ambos com água após cada uso.
Deixe que sequem ao ar; posteriormente, volte a colocar a seringa dosificadora oral junto com a solução oral no envoltório.
Guarde a seringa dosificadora oral com a solução oral de XELJANZ.
Não jogue a seringa dosificadora oral.
- País de registo
- Substância ativa
- Requer receita médicaSim
- Fabricante
- Esta informação é apenas para referência e não constitui aconselhamento médico. Consulte sempre um médico antes de tomar qualquer medicamento. A Oladoctor não se responsabiliza por decisões médicas baseadas neste conteúdo.
- Alternativas a XELJANZ 1 mg/mL SOLUÇÃO ORALForma farmacêutica: COMPRIMIDO, 10 mgSubstância ativa: tofacitinibFabricante: Pfizer Europe Ma EeigRequer receita médicaForma farmacêutica: COMPRIMIDO DE LIBERTAÇÃO MODIFICADA, 11 mgSubstância ativa: tofacitinibFabricante: Pfizer Europe Ma EeigRequer receita médicaForma farmacêutica: COMPRIMIDO, 5 mgSubstância ativa: tofacitinibFabricante: Pfizer Europe Ma EeigRequer receita médica
Alternativas a XELJANZ 1 mg/mL SOLUÇÃO ORAL noutros países
As melhores alternativas com o mesmo princípio ativo e efeito terapêutico.
Alternativa a XELJANZ 1 mg/mL SOLUÇÃO ORAL em Ukraine
Médicos online para XELJANZ 1 mg/mL SOLUÇÃO ORAL
Avaliação de posologia, efeitos secundários, interações, contraindicações e renovação da receita de XELJANZ 1 mg/mL SOLUÇÃO ORAL – sujeita a avaliação médica e regras locais.








