Como usar VYVGART 1000 mg Solução Injetável
Introdução
Prospecto: informação para o paciente
Vyvgart 1 000 mg solução injetável
efgartigimod alfa
Este medicamento está sujeito a acompanhamento adicional, o que agilizará a detecção de nova informação sobre a sua segurança. Pode contribuir comunicando os efeitos adversos que possa ter. A parte final da seção 4 inclui informação sobre como comunicar estes efeitos adversos.
Leia todo o prospecto atentamente antes de começar a usar este medicamento, porque contém informação importante para si.
- Conserva este prospecto, porque pode ter que voltar a lê-lo.
- Se tiver alguma dúvida, consulte o seu médico, farmacêutico ou enfermeiro.
- Este medicamento foi prescrito apenas para si, e não deve dá-lo a outras pessoas, mesmo que tenham os mesmos sintomas que si, porque pode prejudicá-las.
- Se experimentar efeitos adversos, consulte o seu médico, farmacêutico ou enfermeiro, mesmo que se trate de efeitos adversos que não aparecem neste prospecto. Ver seção 4.
Conteúdo do prospecto
- O que é Vyvgart e para que é utilizado
- O que precisa saber antes de começar a usar Vyvgart
- Como usar Vyvgart
- Possíveis efeitos adversos
- Conservação de Vyvgart
- Conteúdo do envase e informação adicional
1. O que é Vyvgart e para que é utilizado
O que é Vyvgart
Vyvgart contém o princípio ativo efgartigimod alfa. Efgartigimod alfa liga-se a uma proteína do organismo denominada receptor neonatal para o Fc (FcRn) e bloqueia-a. Ao bloquear o FcRn, efgartigimod alfa reduz o nível de autoanticorpos frente à imunoglobulina G (IgG), que são proteínas do sistema imunitário que atacam por engano partes do organismo de uma pessoa.
Para que é utilizado Vyvgart
Vyvgart é utilizado juntamente com o tratamento de referência para tratar adultos com miastenia gravis generalizada (MGG), uma doença autoimune que provoca fraqueza muscular. A MGG pode afetar vários grupos musculares de todo o corpo. A doença também pode provocar falta de ar, fadiga extrema e dificuldade para engolir.
Nos pacientes com MGG, os autoanticorpos frente à IgG atacam e danificam proteínas dos nervos denominadas receptores de acetilcolina. Devido a este dano, os nervos não são capazes de contrair os músculos normalmente, o que provoca fraqueza muscular e dificuldade para se mover. Ao ligar-se à proteína FcRn e reduzir os níveis de autoanticorpos, Vyvgart pode melhorar a capacidade de contração dos músculos e reduzir os sintomas da doença e seu impacto nas atividades diárias.
2. O que precisa saber antes de começar a usar Vyvgart
Não use Vyvgart
- se é alérgico a efgartigimod alfa ou a algum dos outros componentes deste medicamento (incluídos na seção 6).
Advertências e precauções
Consulte o seu médico antes de começar a usar Vyvgart.
Classe V de MGFA
O médico não pode prescrever-lhe este medicamento se estiver conectado a um respirador devido à fraqueza muscular por MGG (crise miasténica).
Infecções
O tratamento com Vyvgart pode reduzir a sua resistência natural às infecções, por isso deve informar o seu médico se tiver alguma infecção antes de começar a usar Vyvgart.
Reações à injeção e reações alérgicas
Vyvgart contém uma proteína que pode provocar em algumas pessoas reações como erupção ou picazón. Vyvgart pode causar uma reação anafiláctica (reação alérgica grave). Se experimentar reações alérgicas como inchaço do rosto, lábios, garganta ou língua que dificulte engolir ou respirar, falta de ar, sensação de perda de consciência ou erupção cutânea durante ou após a injeção, informe o seu médico imediatamente.
Imunizações (vacinas)
Informe o seu médico se lhe foi administrada alguma vacina nas últimas 4 semanas, ou se tem previsto vacinar-se em um futuro próximo.
Crianças e adolescentes
Não administre este medicamento a crianças menores de 18 anos, pois não foi estabelecida a segurança e eficácia de Vyvgart nesta população.
Pacientes de idade avançada
Não são necessárias precauções especiais para o tratamento de pacientes maiores de 65 anos.
Outros medicamentos e Vyvgart
Informe o seu médico se está utilizando, utilizou recentemente ou possa ter que utilizar qualquer outro medicamento.
Gravidez, amamentação e fertilidade
Se está grávida ou em período de amamentação, acredita que possa estar grávida ou tem intenção de engravidar, consulte o seu médico ou farmacêutico antes de utilizar este medicamento.
Condução e uso de máquinas
Não se espera que Vyvgart influencie a capacidade de conduzir ou utilizar máquinas.
Vyvgart contém sódio
Este medicamento contém menos de 1 mmol de sódio (23 mg) por frasco; isto é, é essencialmente “isento de sódio”.
Vyvgart contém polissorbato
Este medicamento contém 2,7 mg de polissorbato 20 em cada frasco, equivalente a 0,4 mg/ml. Os polissorbatos podem causar reações alérgicas. Informe o seu médico se tiver alguma alergia conhecida.
3. Como usar Vyvgart
Siga exatamente as instruções de administração deste medicamento indicadas pelo seu médico ou farmacêutico. Em caso de dúvida, consulte novamente o seu médico ou farmacêutico.
Qual dose de Vyvgart receberá e com que frequência
A dose recomendada é de 1 000 mg administrados em ciclos de uma injeção por semana durante 4 semanas. O seu médico decidirá quando são necessários mais ciclos de tratamento.
Se já está em tratamento com Vyvgart por via intravenosa e deseja mudar para Vyvgart por via subcutânea, deverá receber a injeção subcutânea em vez da sua perfusão intravenosa no início do próximo ciclo de tratamento.
Injeção de Vyvgart
Vyvgart é administrado mediante uma injeção debaixo da pele (por via subcutânea). O senhor e o seu médico devem decidir se, após uma formação adequada, o senhor ou o seu cuidador podem injetar Vyvgart. A primeira autoinjeção deve ser realizada diante do seu profissional de saúde. É importante que não tente injetar Vyvgart antes de ter recebido formação por parte de um profissional de saúde.
Se o senhor ou o seu cuidador injetam Vyvgart, o senhor ou o seu cuidador devem ler atentamente e seguir as Instruções de administração que figuram no final deste prospecto (ver “Instruções de uso importantes”). Fale com o seu médico, farmacêutico ou enfermeiro se tiver alguma dúvida sobre como administrar uma injeção.
Se usar mais Vyvgart do que deve
Como Vyvgart é administrado em um frasco de uso único, é pouco provável que receba demasiada quantidade. No entanto, se estiver preocupado, entre em contato com o seu médico, farmacêutico ou enfermeiro para que o aconselhe.
Se perder ou esquecer uma consulta para receber Vyvgart
Leve um registo da sua próxima dose. É importante que use Vyvgart exatamente como lhe foi prescrito pelo seu médico.
- Se esqueceu de tomar a sua dose nos três dias seguintes à data em que devia tomá-la, tome a sua dose assim que se lembrar e, em seguida, siga com a posologia original.
- Se esqueceu de tomar a sua dose durante mais de três dias, pergunte ao seu médico quando deve tomar a próxima dose.
- Se esqueceu de uma consulta, entre em contato com o seu médico imediatamente para que o aconselhe.
Não tome uma dose dupla para compensar as doses esquecidas.
Se interromper o tratamento com Vyvgart
A interrupção ou o cessar do tratamento com Vyvgart pode provocar a reaparição dos seus sintomas de MGG. Consulte com o seu médico antes de interromper o tratamento com Vyvgart. O seu médico explicará os possíveis efeitos adversos e riscos. O seu médico também querrá supervisioná-lo estreitamente.
Se tiver alguma outra dúvida sobre o uso deste medicamento, pergunte ao seu médico, farmacêutico ou enfermeiro.
4. Possíveis efeitos adversos
Como todos os medicamentos, este medicamento pode produzir efeitos adversos, embora nem todas as pessoas os sofram.
O seu médico explicará os possíveis efeitos adversos e os riscos e benefícios de Vyvgart antes do tratamento.
Informe o seu médico imediatamente se notar:
Signos de uma reação alérgica grave (reação anafiláctica) como inchaço do rosto, lábios, garganta ou língua que dificulte engolir ou respirar, falta de ar, sensação de perda de consciência ou erupção cutânea durante ou após a injeção.
Se não estiver seguro de quais são os efeitos adversos que se indicam a seguir, peça ao seu médico que os explique.
Muito frequentes(podem afetar mais de 1 de cada 10 pacientes)
- infecções do nariz e da garganta (vias respiratórias altas)
- reações na zona de injeção, que podem incluir vermelhidão, picazón, dor. Estas reações na zona de injeção são geralmente leves a moderadas e geralmente aparecem um dia após a injeção.
Frequentes(podem afetar até 1 de cada 10 pacientes)
- dor ou sensação de ardor ao urinar, que pode ser um sinal de infecção urinária
- inflamação das vias respiratórias nos pulmões (bronquite)
- dor muscular (mialgia)
- náuseas.
Frequência não conhecida(não pode ser estimada a partir dos dados disponíveis)
- reações alérgicas durante ou após a injeção.
- inchaço do rosto, lábios, garganta ou língua que dificulte engolir ou respirar, falta de ar.
- pele pálida, pulso débil e rápido, ou sensação de perda de consciência.
- erupção repentina, picazón, ou habões.
Comunicação de efeitos adversos
Se experimentar qualquer tipo de efeito adverso, consulte o seu médico, mesmo que se trate de possíveis efeitos adversos que não aparecem neste prospecto. Também pode comunicá-los diretamente através do sistema nacional de notificação incluído no Apêndice V. Mediante a comunicação de efeitos adversos, pode contribuir para fornecer mais informação sobre a segurança deste medicamento.
5. Conservação de Vyvgart
Mantenha este medicamento fora da vista e do alcance das crianças.
Não utilize este medicamento após a data de validade que aparece na caixa e na etiqueta após “CAD”. A data de validade é o último dia do mês que se indica.
Conservar na geladeira (entre 2 °C e 8 °C). Não congelar.
Em caso necessário, os frascos não abertos podem ser conservados à temperatura ambiente (até 30 °C) durante um máximo de 3 dias. Após a conservação à temperatura ambiente, os frascos não abertos podem ser devolvidos à geladeira. O tempo total fora da geladeira e à temperatura ambiente não deve exceder os 3 dias.
Conservar no embalagem original para protegê-lo da luz.
Não utilize este medicamento se observar partículas.
Os medicamentos não devem ser jogados nos desagües nem na lixeira. Pergunte ao seu farmacêutico como se livrar dos envases e dos medicamentos que já não precisa. Desta forma, ajudará a proteger o meio ambiente.
6. Conteúdo do envase e informação adicional
Composição de Vyvgart
- O princípio ativo é efgartigimod alfa. Cada frasco contém 1 000 mg de efgartigimod alfa em 5,6 ml. Cada ml contém 180 mg de efgartigimod alfa.
- Os outros componentes são: hialuronidase humana recombinante (rHuPH20), L-histidina, cloridrato de L-histidina monoidratado, L-metionina, polissorbato 20, cloreto de sódio, sacarose, água para preparações injetáveis. Ver seção 2 “Vyvgart contém sódio”.
Aspecto do produto e conteúdo do envase
Vyvgart é uma solução pronta para uso, ligeiramente amarela, transparente a ligeiramente turva, que se apresenta como solução injetável subcutânea.
Título da autorização de comercialização e responsável pela fabricação
argenx BV
Industriepark-Zwijnaarde 7
9052 Gent
Bélgica
Podem solicitar mais informações sobre este medicamento dirigindo-se ao representante local do titular da autorização de comercialização:
Bélgica/Bélgica/Bélgica/Estônia argenx BV Tel: +32 (0) 93969394/+32 (0) 800 54477 | Lituânia argenx BV Tel: 8 800 80 052 |
| Luxemburgo/Luxemburgo argenx BV Tel: 800 25 233 |
República Tcheca argenx BV Tel: 800 040 854 | Hungria argenx BV Tel: (80) 088 578 |
Dinamarca argenx BV Tlf: 80 25 41 88 | Malta argenx BV Tel: 8006 5101 |
Alemanha argenx Alemanha GmbH Tel: 08001803963 | Países Baixos argenx BV Tel: 0800 0232882 |
Grécia Medison Pharma Grécia Single Member Societe Anonyme Tel: +30 210 0100 188 | Noruega argenx BV Tlf: 800 62 225 |
Espanha argenx Espanha S.L. Tel: 900 876 188 | Áustria argenx BV Tel: 0800 017936 |
França argenx França SAS Tel: +33 (0) 188898992 | Polônia argenx BV Tel: 800 005 155 |
Croácia argenx BV Tel: 800 806 524 | Portugal argenx BV Tel: 800 180 844 |
Irlanda/Reino Unido (Irlanda do Norte) argenx BV Tel: 1800 851 868 | Romênia argenx BV Tel: 0800 360 912 |
Islândia argenx BV Sími: 800 4422 | Eslovênia argenx BV Tel: 0800 688955 |
Itália argenx Itália s.r.l Tel: 800729052 | República Eslovaca argenx BV Tel: 0800 002 646 |
Chipre argenx BV Tel: 80 077122 | Finlândia argenx BV Puh/Tel: 0800 412838 |
Letônia argenx BV Tel: 80 205 267 | Suécia argenx BV Tel: 020-12 74 56 |
Data da última revisão deste prospecto:
Outras fontes de informação
A informação detalhada deste medicamento está disponível no site da Agência Europeia de Medicamentos: https://www.ema.europa.eu. Também existem links para outros sites sobre doenças raras e medicamentos órfãos.
--------------------------------------------------------------------------------------------------------------------Instruções de uso importantes
Vyvgart 1 000 mg solução injetável
efgartigimod alfa
Via subcutânea
Leia e compreenda estas instruções de uso antes de aplicar a injeção de Vyvgart.
Se você ou seu cuidador estiverem dispostos a administrar Vyvgart, seu profissional de saúde os indicará como injetá-lo. Seu profissional de saúde deve ensinar a você ou a seu cuidador como preparar e aplicar a injeção de Vyvgart corretamente antes de usá-lo pela primeira vez. É necessário uma demonstração de autoadministração adequada sob a supervisão de um profissional de saúde. É importante que não tente se injetar o medicamento até que tenha recebido treinamento e você ou seu cuidador estejam seguros de que sabem como usar Vyvgart. Pergunte a seu profissional de saúde se tiver alguma dúvida.
Informação importante que deve conhecer antes de aplicar uma injeção subcutânea de Vyvgart.
- Apenas por via subcutânea.
- O frasco é de uso único. Nãoguarde os frascos, mesmo que não estejam vazios.
- Nãouse um frasco se observar uma turbidez incomum ou partículas visíveis. O medicamento deve ter um aspecto ligeiramente amarelo, de transparente a ligeiramente turvo.
- Nãoagite o frasco durante a manipulação.
- Nãouse frascos danificados ou sem tampa protetora. Notifique e devolva à farmácia os frascos danificados ou sem tampa.
Conservação de Vyvgart
- Conservar na geladeira (entre 2 °C e 8 °C).
- Nãocongelar.
- Se necessário, os frascos não abertos podem ser conservados à temperatura ambiente (até 30 °C) por um máximo de 3 dias. Após a conservação à temperatura ambiente, os frascos não abertos podem ser devolvidos à geladeira. O tempo total fora da geladeira e à temperatura ambiente não deve exceder 3 dias.
- Conservar no embalagem original para protegê-lo da luz.
- Mantenha este medicamento fora do alcance e da vista das crianças.
| |
1 frasco que contém Vyvgart |
|
Bula e instruções de uso de Vyvgart |
|
Material adicional não incluído Conservar o material adicional à temperatura ambiente em um local seco | |
Toalhetes com álcool |
|
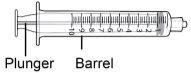
Seringa de 10 ml |
|
Agulha de transferência de calibre 18, ≥ 38 mm de comprimento |
|
Microperfusor de calibre 25, tubo de 30 cm, volume máximo de calibração de 0,4 ml |
|
Gaze estéril |
|
Adesivo adesivo |
|
Contenedor para objetos cortopunzantes |
|
Preparação do material
Passo1 Tirar a caixa do frasco da geladeira. |
|
Passo2 Tirar o frasco da caixa e verificar que:
Se alguma dessas condições não for atendida, nãoadministre a injeção e informe à farmácia. |
|
Passo3 Esperar pelo menos 15 minutos para que o frasco se atempere naturalmente até alcançar a temperatura ambiente. Verificar se o medicamento do frasco é ligeiramente amarelo, de transparente a ligeiramente turvo e não contém partículas visíveis.
|
|
Passo4 Reunir o material adicional seguinte:
| |
Passo5 5a.Limpar a área de trabalho. 5b.Lavar as mãos com sabão e secá-las bem. |
|
Preparação da seringa | |
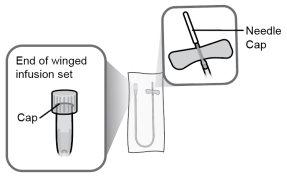
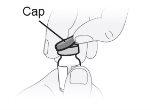
Passo6 Retirar a tampa extraível protetora de plástico do frasco. O lacre de alumínio deve permanecer no lugar. |
|
Passo7 Limpar a tampa de borracha com um toalhete com álcool novo. Deixar secar ao ar de forma natural durante pelo menos 30 segundos. Nãosopre a tampa de borracha. |
|
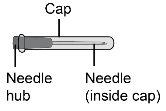
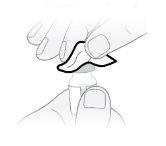
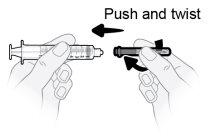
Passo8 Desenvolver a seringa e a agulha de transferência. Introduzir a agulha de transferência na seringa e girar no sentido horário até que a agulha fique firmemente acoplada à seringa. Nãotoque nem a ponta da seringa nem a parte inferior da agulha para evitar germes e risco de infecção. |
|
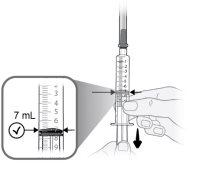
Passo9 Puxar lentamente o êmbolo e introduzir até 7 ml de ar na seringa. |
|
Passo10 10a.Segurar a seringa pelo cone onde a agulha se acopla à seringa. 10b.Segurar o capuchão da agulha de transferência e puxar com cuidado para retirá-lo do corpo. 10c.Colocar o capuchão da agulha de transferência para baixo sobre uma superfície limpa e plana.
|
|
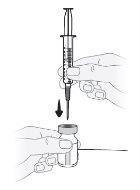
Passo11 Mantenha o frasco em posição vertical sobre uma superfície plana e introduza a agulha de transferência através do centro da tampa de borracha desinfetada. Nãoperfure a tampa de borracha do frasco mais de uma vez para evitar vazamentos. |
|
Passo12 Dê a volta ao frasco mantendo a agulha de transferência introduzida nele. |
|
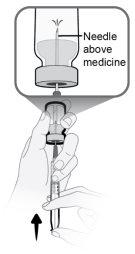
Passo13 13a.Certifique-se de que a agulha de transferência do frasco aponte para cima com a ponta da agulha acima da solução de medicamento. 13b.Empurre suavemente o êmbolo para injetar todo o ar da seringa no espaço vazio sobre a solução de medicamento no frasco. 13c.Mantenha o dedo pressionado sobre o êmbolo da seringa. Nãoinjete ar na solução de medicamento, pois podem ser criadas bolhas de ar ou espuma. |
|
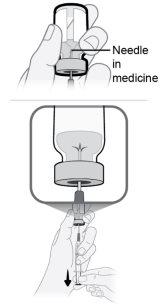
Passo14 Preencha a seringa como segue: 14a.Mantenha o dedo pressionado sobre o êmbolo da seringa e deslize a ponta da agulha de transferência dentro da solução de medicamento no gargalo do frasco (perto da tampa do frasco) de forma que a ponta da agulha fique completamente coberta na solução. 14b.Retire lentamente o êmbolo, mantendo a ponta da agulha de transferência na solução para evitar bolhas de ar e espuma na seringa. Preencha a seringa com todo o conteúdo do frasco. |
|
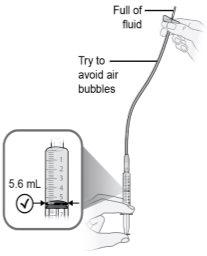
Passo15 Elimine as bolhas de ar grandes, se houver. 15a.Mantenha a agulha de transferência no frasco e verifique se há bolhas de ar grandes na seringa. 15b.Elimine as bolhas de ar grandes dando golpes suaves na seringa com os dedos até que as bolhas subam à parte superior da seringa. 15c.Mova a ponta da agulha de transferência por cima da solução de medicamento e empurre lentamente o êmbolo para cima para expulsar as bolhas de ar da seringa. 15d.Para eliminar qualquer resto de solução de medicamento do frasco, volte a introduzir a ponta da agulha de transferência na solução e puxe lentamente o êmbolo até ter todo o conteúdo do frasco na seringa. 15e.Repita os passos anteriores até que as bolhas de ar grandes sejam eliminadas. Se não for possível extrair todo o líquido do frasco, coloque-o em posição vertical para alcançar a quantidade restante. |
|
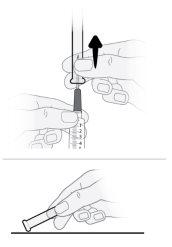
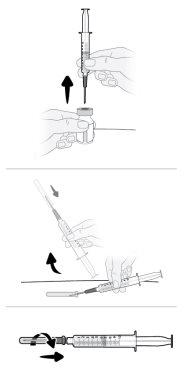
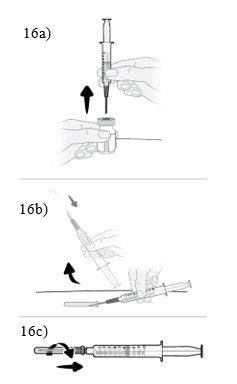
Paso16 16a.Girar o frasco em posição vertical e retirar a seringa e a agulha de transferência do frasco. 16b.Com uma mão, deslizar a agulha de transferência no capuchão e levantar para cobrir a agulha. 16c.Uma vez coberta a agulha de transferência, enroscar o capuchão na seringa para fixá-lo por completo. |
|
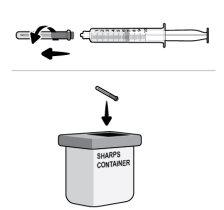
Paso17 17a.Puxar suavemente a agulha de transferência e girá-la no sentido contrário ao das agulhas do relógio para retirá-la da seringa. 17b.Puxar (desechar) a agulha de transferência no contenedor para objetos cortopunzantes. |
|
Preparação da injeção de Vyvgart
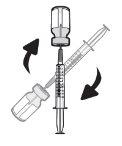
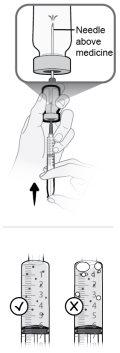
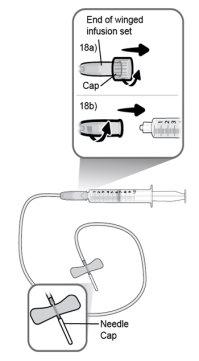
Paso18 18a.Retirar a tampa do extremo do microperfusor. 18b.Empurrar e girar suavemente o extremo do microperfusor no sentido das agulhas do relógio sobre a seringa até que fique firmemente acoplado. A configuração final da seringa deve parecer-se com a figura da direita.
|
|
Paso19 19a.Encher o tubo do microperfusor pressionando suavemente o êmbolo da seringa até que esteja na marca de 5,6 ml. Deve-se ver um pouco de líquido no extremo da agulha. 19b.Colocar a seringa e o microperfusor acoplado sobre uma superfície limpa e plana. Nãolimpar o excesso de solução de medicamento expulsada do equipamento de perfusão durante o preenchimento do tubo. |
|
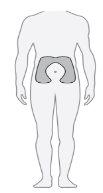
Paso20 Escolher o local de injeção
Escolher um local de injeção diferente cada vez que se injetar (rotar o local) para diminuir as molestias. Nota: Nãoinjetar em zonas onde a pele esteja avermelhada, amoratada, sensível, dura ou em zonas onde haja sardas ou cicatrizes. |
|
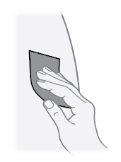
Paso21 Desinfetar o local de injeção com uma toalhita com álcool nova. Realizar movimentos circulares e limpar desde o interior para o exterior. Deixar secar a zona ao ar durante pelo menos 30 segundos. Nãotocar o local de injeção após desinfetá-lo. |
|
Injeção de Vyvgart
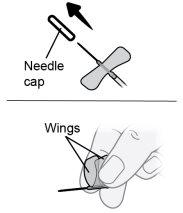
Paso22 22a.Retirar com cuidado o capuchão da agulha do microperfusor. 22b.Dobrar as asas do equipamento de perfusão para cima e segurá-las entre os dedos polegar e indicador, com a agulha por baixo das asas. Nota: Para evitar infecções, certifique-se de que a agulha não entre em contato com nada antes da inserção cutânea. |
|
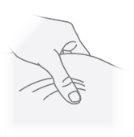
Paso23 Com a mão livre, puxar um dobra da pele ao redor do local de injeção desinfetado e levantá-lo para cima. Pegar suficiente pele para criar uma “carpa” na qual introduzir a agulha. Nãosegurar a pele com muita força para evitar que se formem hematomas. |
|
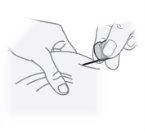
Paso24 Introduzir a agulha no centro da zona de pele puxada em um ângulo de uns 45 graus. Nota: A agulha deve ser introduzida suavemente na pele. Se notar resistência, pode puxar ligeiramente a agulha para trás. |
|
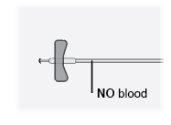
Paso25 Verificar o equipamento de perfusão. Certificar-se de que não há sangue. Importante: Se vir sangue, puxar ligeiramente a agulha para trás sem retirá-la da pele. |
|
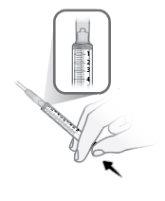
Paso26 Administrar a injeção empurrando o êmbolo da seringa com uma pressão constante até que não reste medicamento na seringa. Isso corresponde à injeção da dose recomendada de 5,6 ml. A injeção geralmente dura entre 30 e 90 segundos. Nota:
|
|
Paso27 27a.Uma vez injetada toda a solução, retirar a agulha da pele. 27b.Cobrir o local de injeção com um adesivo estéril, como um adesivo adesivo. Nota: Se se observar uma pequena gota de sangue após retirar a agulha, nãose preocupe. Isso pode ocorrer se a agulha picar a pele durante a extração. Limpe o sangue com uma gaze estéril e pressione suavemente. Não devem ocorrer mais hemorragias. Aplique um adesivo estéril para cobrir a zona. |
|
Eliminação de Vyvgart
Paso28 Desechar (descartar) o microperfusor (com a agulha e a seringa acopladas) e o frasco no contenedor para objetos cortopunzantes. Se nãodispuser de um contenedor para descartar objetos cortopunzantes, pode utilizar um contenedor doméstico se este:
Desechar o contenedor cheio seguindo as instruções do seu médico ou farmacêutico. Nota: Mantenha sempre o contenedor para objetos cortopunzantes fora da vista e do alcance das crianças. |
|
- País de registo
- Substância ativa
- Requer receita médicaSim
- Fabricante
- Esta informação é apenas para referência e não constitui aconselhamento médico. Consulte sempre um médico antes de tomar qualquer medicamento. A Oladoctor não se responsabiliza por decisões médicas baseadas neste conteúdo.
- Alternativas a VYVGART 1000 mg Solução InjetávelForma farmacêutica: INJETÁVEL, 1000 mgSubstância ativa: efgartigimod alfaFabricante: Argenx B.V.Requer receita médicaForma farmacêutica: PERFURAÇÃO INJETÁVEL, 20 mg/mlSubstância ativa: efgartigimod alfaFabricante: Argenx B.V.Requer receita médicaForma farmacêutica: COMPRIMIDO, 180 mgSubstância ativa: mycophenolic acidFabricante: Laboratorio Stada S.L.Requer receita médica
Alternativas a VYVGART 1000 mg Solução Injetável noutros países
As melhores alternativas com o mesmo princípio ativo e efeito terapêutico.
Alternativa a VYVGART 1000 mg Solução Injetável em Polónia
Médicos online para VYVGART 1000 mg Solução Injetável
Avaliação de posologia, efeitos secundários, interações, contraindicações e renovação da receita de VYVGART 1000 mg Solução Injetável – sujeita a avaliação médica e regras locais.





 Conteúdo do envase
Conteúdo do envase