
VARILRIX pó e solvente para solução injetável em seringa pré-carregada

Pergunte a um médico sobre a prescrição de VARILRIX pó e solvente para solução injetável em seringa pré-carregada

Como usar VARILRIX pó e solvente para solução injetável em seringa pré-carregada
Introdução
Prospecto: informação para o utilizador
Varilrix pó e diluente para solução injetável em seringa pré-carregada
Vacina antivaricela (vírus vivos)
Leia todo o prospecto atentamente antes de si ou seu filho receber este medicamento, porque contém informações importantes para si/seu filho.
- Conserva este prospecto, porque pode ter que voltar a lê-lo.
- Se tiver alguma dúvida, consulte o seu médico, farmacêutico ou enfermeiro.
- Esta vacina foi-lhe prescrita apenas a si ou a seu filho, e não deve dá-la a outras pessoas.
- Se si ou seu filho experimentar efeitos adversos, consulte o seu médico, farmacêutico ou enfermeiro, mesmo que se trate de efeitos adversos que não aparecem neste prospecto. Ver seção 4.
Conteúdo do prospecto
- O que é Varilrix e para que é utilizado
- O que precisa saber antes de si ou seu filho receber Varilrix
- Como é administrado Varilrix
- Possíveis efeitos adversos
- Conservação de Varilrix
- Conteúdo do envase e informação adicional
1. O que é Varilrix e para que é utilizado
Varilrix é uma vacina para uso em pessoas a partir dos 12 meses de idade para protegê-las contra a varicela. Em algumas circunstâncias, Varilrix também pode ser administrado a lactentes a partir dos 9 meses de idade.
A vacinação dentro dos 3 dias posteriores ao contacto com um caso de varicela pode ajudar a prevenir a varicela ou a reduzir a gravidade da doença.
Como funciona Varilrix
Quando se vacina uma pessoa com Varilrix, o sistema imunológico (o sistema de defesa natural do corpo) produzirá anticorpos para proteger a pessoa da infecção pelo vírus da varicela. Varilrix contém vírus debilitados, por isso é muito improvável que cause a varicela em pessoas saudáveis.
Assim como com qualquer vacina, pode ser que Varilrix não proteja completamente todas as pessoas vacinadas.
2. O que precisa saber antes de si ou seu filho receber Varilrix
Não useVarilrix
- Se si ou seu filho padece alguma doença (como distúrbios sanguíneos, cancro, infecção pelo Vírus da Imunodeficiência Humana (VIH) ou Síndrome da Imunodeficiência Adquirida (SIDA)) ou está tomando algum medicamento (incluindo doses altas de corticosteroides) que possa debilitar o sistema imune. Que si ou seu filho receba a vacina vai depender do nível das suas defesas. Ver seção 2 “Advertências e precauções”.
- Se si ou seu filho é alérgico a algum dos componentes desta vacina (incluídos na seção 6). Os sinais de uma reação alérgica podem incluir: erupção cutânea com picazón, dificuldade para respirar e inchação da face ou língua.
- Se si ou seu filho é alérgico à neomicina (um antibiótico). Os antecedentes de dermatite de contacto (erupção cutânea produzida quando a pele entra em contacto direto com alérgenos como a neomicina) não devem ser uma razão para não se vacinar. No entanto, consulte primeiro o seu médico.
- Se si ou seu filho teve previamente uma reação alérgica a qualquer vacina contra a varicela.
- Se si ou sua filha está grávida. Além disso, deve evitar a gravidez durante 1 mês após a vacinação.
Advertências e precauções
Consulte o seu médico, farmacêutico ou enfermeiro antes de si ou seu filho receber Varilrix:
- Se si ou seu filho padece uma infecção grave com febre alta. Pode ser necessário adiar a vacinação até a sua recuperação. Uma infecção de pouca importância, como um resfriado, não requer adiar a vacinação. No entanto, consulte primeiro com o seu médico.
- Se si ou seu filho tem um sistema imunitário debilitado devido a doenças (p. ex., infecção por VIH) e/ou tratamentos. Si ou seu filho deve ser vigilado estreitamente, pois a resposta à vacinação pode não ser suficiente para assegurar uma proteção contra a doença (ver seção 2 “Não use Varilrix”).
- se si padece problemas de sangramento ou lhe saem nódoas negras com facilidade.
Antes ou depois de qualquer injeção, pode produzir-se um desmaio (especialmente em adolescentes), por isso deve informar o seu médico ou enfermeiro se si ou seu filho se desmaiou em ocasiões anteriores após a administração de uma injeção.
Assim como com outras vacinas, pode ser que Varilrix não o proteja completamente a si ou a seu filho contra a varicela. No entanto, as pessoas que foram vacinadas e desenvolvem a varicela têm, normalmente, uma doença muito leve em comparação com as pessoas que não foram vacinadas.
Em raros casos, o vírus debilitado pode transmitir-se de uma pessoa vacinada para outras. Normalmente, isso acontece quando a pessoa vacinada tem algumas manchas ou bolhas na pele. As pessoas saudáveis que se infectam desta forma só desenvolvem, normalmente, uma erupção cutânea leve que não é prejudicial.
Uma vez vacinado, si ou seu filho deve evitar, na medida do possível, durante 6 semanas após a vacinação, uma relação estreita com as seguintes pessoas:
- pessoas com um sistema imunológico debilitado,
- mulheres grávidas que não sofreram a varicela ou não foram vacinadas contra a varicela,
- lactentes recém-nascidos de mães que não sofreram a varicela ou não foram vacinadas contra a varicela.
Outros medicamentos e Varilrix
Informa ao seu médico ou farmacêutico se si ou seu filho está tomando, tomou recentemente ou pode ter que tomar qualquer outra vacina e/ou medicamento.
Informa ao seu médico se si ou seu filho tem que fazer um teste cutâneo para detectar uma possível tuberculose. Se este teste for realizado dentro das 6 semanas posteriores à administração de Varilrix, pode ser que o resultado não seja fiável.
Deve adiar a vacinação durante pelo menos 3 meses se si ou seu filho recebeu uma transfusão de sangue ou anticorpos humanos (imunoglobulinas).
Deve evitar o uso de aspirina ou outros salicilatos (uma substância presente em alguns medicamentos usados para reduzir a febre e aliviar a dor) durante 6 semanas após a vacinação com Varilrix, pois isso pode produzir uma doença grave chamada Síndrome de Reye que pode afetar todos os órgãos do corpo.
Varilrix pode ser administrado ao mesmo tempo que outras vacinas. Será empregado um local de injeção distinto para cada vacina.
Gravidez e lactação
Varilrix não deve ser administrado a mulheres grávidas.
Se si ou sua filha está grávida ou em período de lactação, acredita que possa estar grávida ou tem intenção de ficar grávida, consulte o seu médico ou farmacêutico antes de lhe administrarem a vacina. Também é importante que si ou sua filha não fique grávida durante um mês após a vacinação. Durante este tempo, deve usar um método anticonceptivo eficaz para evitar a gravidez.
Informa ao seu médico se si ou sua filha está a amamentar ou se tem intenção de o fazer. O seu médico decidirá se a si ou a sua filha devem administrar Varilrix.
Condução e uso de máquinas
Varilrix tem uma influência nula ou insignificante sobre a capacidade para conduzir e utilizar máquinas. No entanto, alguns dos efeitos mencionados na seção 4 "Possíveis efeitos adversos" podem afetar de forma temporal a capacidade para conduzir ou utilizar máquinas.
Varilrix contém sorbitol e fenilalanina
Esta vacina contém 6 mg de sorbitol em cada unidade de dose.
Esta vacina contém 331 microgramas de fenilalanina em cada unidade de dose. A fenilalanina pode ser prejudicial em caso de padecer fenilcetonúria (FCN), uma doença genética rara em que a fenilalanina se acumula devido a que o organismo não é capaz de eliminá-la corretamente.
3. Como é administrado Varilrix
Varilrix é injetado sob a pele ou num músculo, seja na parte superior do braço ou na parte exterior da coxa.
As pessoas a partir dos 12 meses de idade devem receber 2 doses de Varilrix com pelo menos 6 semanas de diferença. O tempo entre a primeira e a segunda dose não deveser inferior a 4 semanas.
Em algumas circunstâncias, pode ser administrada a primeira dose de Varilrix a bebês de 9 a 11 meses de idade. Nesses casos, são necessárias duas doses e devem ser administradas com, pelo menos, 3 meses de diferença.
As pessoas que têm risco de varicela grave, como as que recebem tratamento para o cancro, podem receber doses adicionais. O intervalo entre doses não deveser inferior a 4 semanas.
O seu médico determinará o momento e o número de doses apropriados com base nas recomendações oficiais correspondentes.
Se si ou seu filho receber mais Varilrix do que deve
A sobredose é muito pouco provável porque a vacina é fornecida em um frasco de dose única e é administrada por um médico ou enfermeiro. No entanto, se si ou seu filho receber mais Varilrix do que deve, consulte imediatamente o seu médico, farmacêutico ou enfermeiro ou ligue para o Serviço de Informação Toxicológica, telefone 91 562 04 20.
Foram notificados poucos casos de administração acidental e apenas em alguns deles foram notificados sonolência anormal e ataques (convulsões).
Se acredita que si ou seu filho esqueceu de receber uma dose de Varilrix
Entre em contato com o seu médico, que decidirá se é necessário uma dose e quando administrá-la.
4. Possíveis efeitos adversos
Assim como com todos os medicamentos, este medicamento pode produzir efeitos adversos, embora nem todas as pessoas os sofram.
Podem produzir-se os seguintes efeitos adversos com esta vacina:
- Muito frequentes (podem afetar mais de 1 de cada 10 pessoas):
- dor e vermelhidão no local da injeção
- Frequentes (podem afetar até 1 de cada 10 pessoas):
- erupção cutânea (manchas e/ou bolhas)
- inchação no local da injeção*
- febre igual ou superior a 38ºC (rectal)*
- Pouco frequentes (podem afetar até 1 de cada 100 pessoas):
- infecção das vias respiratórias altas
- dor de garganta e molestias ao engolir (faringite)
- inchação dos gânglios linfáticos
- irritabilidade
- dor de cabeça
- sonolência
- tosse
- picazón, secreção ou congestão nasal, espirros (rinite)
- náuseas
- vómitos
- erupção semelhante à varicela
- picazón
- dor nas articulações
- dor nos músculos
- febre superior a 39,5 ºC (rectal)
- falta de energia (cansação)
- malestar geral
- Raros (podem afetar até 1 de cada 1000 pessoas):
- inflamação do olho (conjuntivite)
- dor de estômago
- diarreia
- erupção abultada com picazón (urticária)
*A inchação no local da injeção e a febre podem ocorrer muito frequentemente em adolescentes e em adultos. A inchação também pode ocorrer muito frequentemente após a segunda dose em crianças menores de 13 anos de idade.
Foram notificados os seguintes efeitos adversos em algumas ocasiões, durante o uso rotineiro de Varilrix:
- herpes zóster
- pequenas manchas hemorrágicas ou aparecimento de nódoas negras com mais facilidade do que o normal devido à diminuição de um tipo de células do sangue chamadas plaquetas
- reações alérgicas. Erupções que podem causar picazón ou bolhas, inchação dos olhos e da face, dificuldade para respirar ou engolir, queda repentina da pressão sanguínea e perda da consciência. É possível que estas reações se produzam antes de abandonar a consulta do médico. No entanto, se si ou seu filho desenvolver qualquer um destes sintomas, entre em contato urgentemente com um médico
- infecção ou inflamação do cérebro, medula espinhal e nervos periféricos que produz dificuldade temporária para caminhar (inestabilidade) e/ou perda temporária do controlo dos movimentos corporais, acidente vascular cerebral (dano cerebral produzido por uma interrupção do fluxo de sangue)
- ataques ou convulsões
- inflamação, estreitamento ou obstrução dos vasos sanguíneos. Isso pode implicar sangramento incomum ou aparecimento de nódoas negras debaixo da pele (púrpura de Henoch-Schönlein) ou febre com uma duração superior a cinco dias associada a uma erupção no tronco seguida, por vezes, de uma descamação da pele em mãos e dedos, vermelhidão dos olhos, dos lábios, da garganta e da língua (doença de Kawasaki)
- eritema multiforme (os sintomas são manchas vermelhas, muitas vezes com picazón, semelhantes à erupção do sarampo, que começa nas extremidades e, por vezes, na face e no resto do corpo).
Comunicação de efeitos adversos
Se si ou seu filho experimentar qualquer tipo de efeito adverso, consulte o seu médico, farmacêutico ou enfermeiro, mesmo que se trate de possíveis efeitos adversos que não aparecem neste prospecto. Também pode comunicá-los diretamente através do Sistema Espanhol de Farmacovigilância de Medicamentos de Uso Humano, https://www.notificaram.es. Mediante a comunicação de efeitos adversos, si pode contribuir para fornecer mais informações sobre a segurança deste medicamento.
5. Conservação de Varilrix
Mantenha esta vacina fora da vista e do alcance das crianças.
Não utilize esta vacina após a data de validade que aparece no envase. A data de validade é o último dia do mês que se indica.
Conservar e transportar em frigorífico (entre 2º C e 8º C).
Conservar no embalagem original para protegê-la da luz.
Depois da reconstituição, a vacina deve ser administrada imediatamente.
Se não for possível, a vacina reconstituída pode ser mantida até 90 minutos a temperatura ambiente (25º C) ou até 8 horas em frigorífico (entre 2º C e 8º C). Se não for utilizada dentro dos prazos e das condições de armazenamento em uso recomendados, a vacina reconstituída deve ser descartada.
Os medicamentos não devem ser jogados nos esgotos nem na lixeira. Deposite os envases e os medicamentos que não precisa no Ponto SIGRE da farmácia. Em caso de dúvida, pergunte ao seu farmacêutico como se livrar dos envases e dos medicamentos que já não precisa. Desta forma, ajudará a proteger o meio ambiente.
6. Conteúdo do envase e informação adicional
Composição de Varilrix
- O princípio ativo é: vírus vivos atenuados da varicela (cepa Oka, produzidos em
células diploides humanas MRC-5). Cada dose de 0,5 ml da vacina reconstituída contém não menos de 10^3,3 UFP (unidades formadoras de placa) do vírus da varicela.
- Os demais componentes são:
Pó: aminoácidos (que contêm fenilalanina), lactose anidra, sorbitol (E-420), manitol (E-421).
Veículo: água para preparações injetáveis.
Aspecto do produto e conteúdo do envase
Varilrix apresenta-se como um pó e veículo para solução injetável (pó em um frasco para 1 dose e veículo em uma seringa pré-carregada (0,5 ml), com ou sem agulhas separadas nos seguintes tamanhos de embalagem:
- com 1 agulha separada: tamanhos de embalagem de 1 ou 10.
- com 2 agulhas separadas: tamanhos de embalagem de 1 ou 10.
- sem agulhas: tamanhos de embalagem de 1 ou 10.
Varilrix é fornecido como um pó de cor creme clara a amarelada ou rosácea e um veículo incolor e transparente (água para preparações injetáveis) para reconstituir a vacina.
Pode ser que apenas alguns tamanhos de embalagens sejam comercializados.
Título de autorização de comercialização e responsável pela fabricação
Título de autorização de comercialização
GlaxoSmithKline, S.A.
PTM C/ Severo Ochoa 2
28760 - Tres Cantos
Madrid
Telefone: 900 202 700
Fax: 91 807 03 10
e-mail: [email protected]
Responsável pela fabricação
GlaxoSmithKline Biologicals
Rue de l´Institut 89
1330 Rixensart (Bélgica)
Este medicamento está autorizado nos estados membros do Espaço Económico Europeucom os seguintes nomes:
Estado membro | Nome |
Alemanha, Áustria, Bélgica, Chipre, Dinamarca, Estónia, Finlândia, França, Grécia, Hungria, Islândia, Itália, Luxemburgo, Malta, Noruega, Polónia, Portugal, República Checa, Roménia, Suécia | VARILRIX |
Espanha | VARILRIX pó e veículo para solução injetável em seringa pré-carregada |
Letónia | Varilrix pulveris un šķīdinātājs injekciju šķīduma pagatavošanai pilnšļirce |
Lituânia | Varilrix milteliai ir tirpiklis injekciniam tirpalui užpildytame švirkšte |
Data da última revisão deste prospecto:05/2025
Outras fontes de informação
A informação detalhada e atualizada deste medicamento está disponível na página Web da Agência Espanhola de Medicamentos e Produtos Sanitários (AEMPS) http://www.aemps.gob.es/.
-------------------------------------------------------------------------------------------------------------
Esta informação está destinada apenas a profissionais do setor sanitário:
Assim como com todas as vacinas injetáveis, deve existir uma vigilância médica adequada e dispor de tratamento médico em caso de que se produzisse uma reação anafiláctica infrequente após a administração da vacina.
Deve-se permitir que o álcool e outros agentes antisépticos se evaporem da pele antes de injetar a vacina, pois podem inativar os vírus atenuados na vacina.
Varilrix não deve ser administrado por via intravascular nem intradérmica.
Na ausência de estudos de compatibilidade, este medicamento não deve ser misturado com outros.
Deve-se inspecionar visualmente o veículo e a vacina reconstituída. A cor da vacina reconstituída pode variar entre laranja claro e rosa devido a pequenas variações do seu pH. Pode conterpartículas translúcidas relacionadas com o produto. Isso é normal e não compromete a ação da vacina.
Não administrar se a vacina apresentar outra coloração ou conter outras partículas.
A vacina deve ser reconstituída adicionando todo o conteúdo da seringa pré-carregada de veículo ao frasco que contém o pó.
Para saber como inserir a agulha na seringa, leia-se atentamente as instruções fornecidas com as imagens 1 e 2. No entanto, a seringa facilitada com Varilrix pode ser ligeiramente diferente (sem rosca de parafuso) da seringa da imagem. Nesse caso, a agulha deve ser inserida sem parafusar.
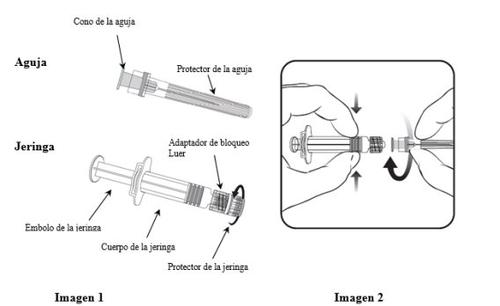
Segurar sempre a seringa pelo corpo, não pelo êmbolo nem pelo adaptador de bloqueio Luer (ABL), e manter a agulha no eixo da seringa (como se mostra na imagem 2). Caso contrário, o ABL poderia deformar-se e causar vazamentos.
Se durante a montagem da seringa o ABL se soltar, usar uma nova dose da vacina (nova seringa e frasco).
- Desparafusar o protetor da seringa girando-o no sentido contrário ao das agulhas do relógio (como se mostra na imagem 1).
Tanto se o ABL gira como se não, por favor, siga os seguintes passos:
- Inserir a agulha na seringa encaixando com delicadeza o cone da agulha no ABL e girar um quarto de volta no sentido das agulhas do relógio até sentir que se bloqueia (como se mostra na imagem 2).
- Retirar o protetor da agulha (pode ser difícil).
- Adicionar o veículo ao pó. Deve-se agitar bem a mistura até que o pó esteja completamente dissolvido.
- Retirar todo o conteúdo do frasco
- Deve-se utilizar uma agulha nova para administrar a vacina. Desparafusar a agulha da seringa e inserir a agulha para a injeção repetindo o passo 2 anterior.
Depois da reconstituição, recomenda-se injetar a vacina o mais rápido possível. No entanto, demonstrou-se que a vacina reconstituída pode ser mantida até 90 minutos a temperatura ambiente (25º C) e até 8 horas na geladeira (entre 2º C e 8º C). Se não for utilizada dentro dos prazos e das condições de armazenamento em uso recomendados, a vacina reconstituída deve ser descartada.
A eliminação da vacina não utilizada e de todos os materiais que tenham estado em contato com ela será realizada de acordo com a normativa local.
- País de registo
- Substância ativa
- Requer receita médicaSim
- Fabricante
- Esta informação é apenas para referência e não constitui aconselhamento médico. Consulte sempre um médico antes de tomar qualquer medicamento. A Oladoctor não se responsabiliza por decisões médicas baseadas neste conteúdo.
- Alternativas a VARILRIX pó e solvente para solução injetável em seringa pré-carregadaForma farmacêutica: INJETÁVEL, mínimo de 1350 UFP/0.5 ml doseSubstância ativa: varicella, live attenuatedFabricante: Schering Plough S.A.Requer receita médicaForma farmacêutica: INJETÁVEL, 50 microgramasSubstância ativa: zoster, purified antigenFabricante: Glaxosmithkline BiologicalsRequer receita médicaForma farmacêutica: INJETÁVEL, 60 microgramas/dose + 60 microgramas/doseSubstância ativa: respiratory syncytial virus vaccinesFabricante: Pfizer Europe Ma EeigRequer receita médica
Alternativas a VARILRIX pó e solvente para solução injetável em seringa pré-carregada noutros países
As melhores alternativas com o mesmo princípio ativo e efeito terapêutico.
Alternativa a VARILRIX pó e solvente para solução injetável em seringa pré-carregada em Polónia
Alternativa a VARILRIX pó e solvente para solução injetável em seringa pré-carregada em Ukraine
Médicos online para VARILRIX pó e solvente para solução injetável em seringa pré-carregada
Avaliação de posologia, efeitos secundários, interações, contraindicações e renovação da receita de VARILRIX pó e solvente para solução injetável em seringa pré-carregada – sujeita a avaliação médica e regras locais.














