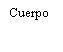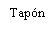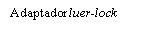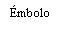
TWINRIX ADULTOS, SUSPENSÃO INJETÁVEL EM SERINGA PREENCHIDA

Pergunte a um médico sobre a prescrição de TWINRIX ADULTOS, SUSPENSÃO INJETÁVEL EM SERINGA PREENCHIDA

Como usar TWINRIX ADULTOS, SUSPENSÃO INJETÁVEL EM SERINGA PREENCHIDA
Introdução
Prospecto: informação para o utilizador
Twinrix Adultos, Suspensão injetável em seringa pré-carregada
Vacina (HAB) (adsorvida) anti-hepatite A (inativada) e anti-hepatite B (ADN)
Leia todo o prospecto atentamente antes de começar a receber esta vacina,pois contém informações importantes para si.
- Conserva este prospecto, pois pode ter que relê-lo.
- Se tiver alguma dúvida, consulte o seu médico ou farmacêutico.
- Esta vacina foi prescrita apenas para si, e não deve dá-la a outras pessoas.
- Se experimentar efeitos adversos, consulte o seu médico ou farmacêutico, mesmo que se trate de efeitos adversos que não aparecem neste prospecto. Ver seção 4.
Conteúdo do prospecto
- O que é Twinrix Adultos e para que é utilizado
- O que precisa saber antes de receber Twinrix Adultos
- Como administrar Twinrix Adultos
- Posíveis efeitos adversos
- Conservação de Twinrix Adultos
- Conteúdo do envase e informação adicional
1. O que é Twinrix Adultos e para que é utilizado
Twinrix Adultos é uma vacina utilizada em adultos e em adolescentes de 16 anos ou mais para prevenir duas doenças: a hepatite A e a hepatite B. A vacina actua fazendo com que o organismo produza a sua própria proteção (anticorpos) contra estas doenças.
- Hepatite A: A hepatite A é uma doença infecciosa que pode afectar o fígado. Esta doença é causada pelo vírus da hepatite A. A hepatite A pode ser transmitida de pessoa para pessoa através de alimentos e bebidas, ou nadando em águas contaminadas por águas residuais. Os sintomas da hepatite A começam de 3 a 6 semanas após o contacto com o vírus. Estes consistem em náuseas (malestar), febre e dores. Após vários dias, o branco dos olhos e a pele podem tornar-se amarelados (icterícia). A gravidade e o tipo de sintomas podem variar. As crianças pequenas podem não desenvolver icterícia. A maioria das pessoas recupera-se completamente, mas geralmente a doença é suficientemente grave para que os pacientes estejam doentes durante aproximadamente um mês.
- Hepatite B: A hepatite B é causada pelo vírus da hepatite B. Causa inflamação do fígado. O vírus encontra-se nos fluidos corporais, tais como o sangue, o sêmen, as secreções vaginais ou a saliva (escarro) de pessoas infectadas.
A vacinação é a melhor forma de se proteger contra estas doenças. Nenhum dos componentes da vacina é infeccioso.
2. O que precisa saber antes de receber Twinrix Adultos
Twinrix Adultos não deve ser administrado se:
- é alérgico a:
- os princípios activos ou a algum dos outros componentes da vacina (incluídos na seção 6)
- a neomicina.
Os sinais de uma reação alérgica podem incluir erupção cutânea com picar, dificuldade para respirar e inflamação da face ou da língua
- teve anteriormente uma reação alérgica a qualquer vacina contra a hepatite A e a hepatite B
- tem uma infecção grave com febre (maior de 38ºC). Uma infecção de pouca importância, como um resfriado, não deveria ser um problema para a vacinação, mas diga ao seu médico.
Advertências e precauções
Consulte o seu médico ou farmacêutico antes de receber Twinrix Adultos se:
- teve algum problema de saúde após a administração prévia de uma vacina
- tem um sistema imunológico debilitado devido a uma doença ou a um tratamento farmacológico
- tem algum problema hemorrágico ou aparecem equimoses com facilidade.
Antes ou depois de qualquer injeção, pode ocorrer um desmaio (especialmente em adolescentes), por isso deve informar o seu médico ou enfermeiro se já desmaiou em ocasiões anteriores após a administração de uma injeção.
Em pessoas obesas, observou-se uma baixa resposta à vacina, possivelmente sem atingir a proteção contra a hepatite A. Também se observou uma baixa resposta à vacina, possivelmente sem atingir a proteção contra a hepatite B, em sujeitos de idade avançada, em homens mais do que em mulheres, em fumadores, em obesos e em pessoas com doenças de longa duração, ou que recebem algum tipo de tratamento farmacológico. O seu médico pode recomendar que se submeta a uma análise de sangue após completar o ciclo de vacinação para verificar se alcançou uma resposta satisfatória. Se não for assim, o seu médico indicará a possibilidade de necessitar de doses adicionais.
Outros medicamentose Twinrix Adultos
Informa o seu médico ou farmacêutico se está utilizando, utilizou recentemente ou pode ter que utilizar qualquer outro medicamento.
Gravidez e amamentação
Se está grávida ou em período de amamentação, acredita que possa estar grávida ou tem intenção de engravidar, consulte o seu médico ou farmacêutico antes de receber esta vacina.
Desconhece-se se Twinrix Adultos passa para o leite materno, no entanto não é de esperar que a vacina cause problemas aos lactentes.
Twinrix Adultos contém neomicina e sódio
Informa o seu médico se já teve uma reação alérgica à neomicina (antibiótico).
Esta vacina contém menos de 1 mmol de sódio (23 mg) por dose; isto é, é essencialmente “isento de sódio”.
3. Como administrar Twinrix Adultos
Receberá um total de três injeções durante um período de 6 meses. Cada injeção será administrada em uma visita independente. A primeira dose será administrada na data escolhida. As outras duas doses serão administradas um mês e seis meses após a primeira dose.
- Primeira dose: na data escolhida
- Segunda dose: 1 mês após
- Terceira dose: 6 meses após a primeira dose
Também pode ser administrada um total de 3 doses de Twinrix Adultos durante 1 mês. Esta pauta de vacinação só pode ser administrada a adultos que necessitem de uma proteção rápida (p. ex. viajantes). A primeira dose será administrada na data escolhida. As outras 2 doses serão administradas 7 e 21 dias após a primeira dose. Recomenda-se uma quarta dose aos 12 meses.
- Primeira dose: na data escolhida
- Segunda dose: 7 dias após
- Terceira dose: 21 dias após a primeira dose
- Quarta dose: 12 meses após a primeira dose
O médico informará se são necessárias doses adicionais e futuras doses de recuerdo.
Como se indica no apartado 2, é mais frequente uma baixa resposta à vacina, possivelmente sem atingir a proteção contra a hepatite B, em sujeitos de idade avançada, em homens mais do que em mulheres, em fumadores, em obesos e em sujeitos com doenças de longa duração, ou que recebem algum tipo de tratamento farmacológico. O seu médico pode recomendar que se submeta a uma análise de sangue após completar o ciclo de vacinação para verificar se alcançou uma resposta satisfatória. Se não for assim, o seu médico indicará a possibilidade de necessitar de doses adicionais.
Se perder uma das injeções previstas, fale com o seu médico para marcar outra visita.
Certifique-se de que completa o ciclo completo de vacinação de três injeções. Caso contrário, pode não estar completamente protegido contra as doenças.
O médico administrará a injeção de Twinrix Adultos no músculo superior do braço.
A vacina não deve ser injetada por via subcutânea (profunda) ou intramuscular na nádega, pois a proteção pode ser menor.
A vacina nunca deve ser injetada em uma veia.
Se tiver alguma outra dúvida sobre o uso desta vacina, pergunte ao seu médico ou farmacêutico.
4. Posíveis efeitos adversos
Como todos os medicamentos, esta vacina pode produzir efeitos adversos, embora nem todas as pessoas os sofram.
Os efeitos adversos que podem ocorrer são os seguintes:
Muito frequentes(podem ocorrer em mais de 1 de cada 10 doses de vacina): dor de cabeça, dor e rubor no local da injeção, cansaço.
Frequentes(podem ocorrer até em 1 de cada 10 doses de vacina): diarreia, náuseas, inflamação, equimoses ou picar no local da injeção, malestar geral.
Pouco frequentes(podem ocorrer até em 1 de cada 100 doses de vacina): tontura, vómitos, dor de estômago, dores musculares, infecção do trato respiratório superior, febre igual ou maior de 37,5°C.
Raros(podem ocorrer até em 1 de cada 1.000 doses de vacina): inflamação das glândulas do pescoço, da axila ou da virilha (linfadenopatia), perda de sensibilidade da pele ao dor ou ao tato (hipoestesia), sensação de formigamento (parestesia), erupção cutânea, picar, dor articular, perda de apetite, pressão sanguínea baixa, sintomas gripais, tais como febre, dor de garganta, gotejamento nasal, tosse e calafrios.
Muito raros(podem ocorrer até em 1 de cada 10.000 doses de vacina):
Entre os efeitos adversos que ocorreram muito raramente durante os ensaios clínicos, o uso rotineiro da vacina ou com vacinas individuais anti-hepatite A e anti-hepatite B incluem: redução das plaquetas, que aumenta o risco de sangramento ou aparência de equimoses (trombocitopenia), manchas roxas ou marrons visíveis através da pele (púrpura trombocitopénica), inflamação ou infecção do cérebro (encefalite), doença degenerativa do cérebro (encefalopatia), inflamação dos nervos (neurite), insensibilidade ou fraqueza dos braços e pernas (neuropatia), paralisia, convulsões ou ataques, inflamação da face, boca ou garganta (edema angioneurótico), inchaço roxo ou roxo-avermelhado da pele (líquen plano), erupções graves da pele (eritema multiforme), habão urticarial, inflamação das articulações, fraqueza muscular, infecção ao redor do cérebro que pode produzir dor de cabeça grave com rigidez de pescoço e sensibilidade à luz (meningite), inflamação de alguns vasos sanguíneos (vasculite), resultados anormais dos testes hepáticos de laboratório, esclerose múltipla, inflamação da medula espinhal (mielite), pálpebras caídas e afundamento dos músculos de um lado da face (paralisia facial), inflamação temporária dos nervos, que causa dor, fraqueza e paralisia dos membros e frequentemente progride para o peito e a face (síndrome de Guillain-Barré), doença dos nervos do olho (neurite óptica), dor imediata no local da injeção, ardor e sensação de queimadura.
Reações alérgicas graves (anafilaxia, reações anafilactoides e reação tipo doença do soro) também podem ocorrer muito raramente (até com 1 de cada 10.000 doses da vacina). Alguns sinais de reações alérgicas graves podem ser erupções cutâneas com picar ou com bolhas, inflamação dos olhos e da face, dificuldade para respirar ou engolir, descenso repentino da pressão sanguínea e perda do conhecimento. Estas reações podem produzir-se antes de abandonar a consulta do médico. Em qualquer caso, se aparecer algum destes sintomas, deve procurar um médico imediatamente.
Comunicação de efeitos adversos
Se experimentar qualquer tipo de efeito adverso, consulte o seu médico ou farmacêutico, mesmo que se trate de possíveis efeitos adversos que não aparecem neste prospecto. Também pode comunicá-los directamente através do Sistema Español de Farmacovigilância de Medicamentos de Uso Humano, https://www.notificaram.es. Mediante a comunicação de efeitos adversos, pode contribuir para fornecer mais informações sobre a segurança deste medicamento.
5. Conservação de Twinrix Adultos
Mantenha este medicamento fora da vista e do alcance das crianças.
Não utilize este medicamento após a data de validade que aparece no envase. A data de validade é o último dia do mês que se indica.
Conservar em frigorífico (entre 2ºC e 8ºC).
Conservar no embalagem original para protegê-lo da luz.
Não congelar. A congelação destrói a vacina.
Os medicamentos não devem ser jogados nos esgotos nem na lixeira. Pergunte ao seu farmacêutico como se livrar dos envases e dos medicamentos que já não precisa. Desta forma, ajudará a proteger o meio ambiente.
6. Conteúdo do frasco e informação adicional
Composição de Twinrix Adultos
Os princípios ativos são:
Vírus da hepatite A (inativados)1,2 720 Unidades ELISA
Antígeno de superfície da hepatite B3,4 20 microgramas
1Produzido em células diploides humanas (MRC-5)
2Adsorvido em hidróxido hidratado de alumínio 0,05 miligramas Al3+
3Produzido pela tecnologia do ADN recombinante em células de levedura (Saccharomyces cerevisiae)
4Adsorvido em fosfato de alumínio 0,4 miligramas Al3+
Os demais componentes de Twinrix Adultos são: cloreto de sódio e água para preparações injetáveis.
Aspecto deTwinrix Adultose conteúdo do frasco
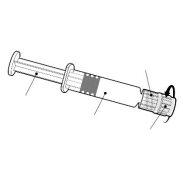
Suspensão injetável em seringa pré-carregada.
Twinrix Adultos é um líquido branco, ligeiramente leitoso.
Twinrix Adultos está disponível em seringa pré-carregada de 1 dose com ou sem agulhas separadas, tamanhos de embalagem de 1, 10 e 25.
Pode ser que apenas alguns tamanhos de embalagens estejam comercializados.
Título da autorização de comercialização e responsável pela fabricação
GlaxoSmithKline Biologicals s.a.
Rue de l’Institut 89
B-1330 Rixensart
Bélgica
Podem solicitar mais informações sobre este medicamento dirigindo-se ao representante local do titular da autorização de comercialização:
Bélgica GlaxoSmithKline Pharmaceuticals SA/NV Tel: + 32 10 85 52 00 | Lituânia GlaxoSmithKline Biologicals SA Tel: +370 80000334 |
Bulgária GlaxoSmithKline Biologicals SA Tel: + 359 80018205 | Luxemburgo GlaxoSmithKline Pharmaceuticals SA/NV Tel: + 32 10 85 52 00 |
República Tcheca GlaxoSmithKline s.r.o. Tel: + 420 2 22 00 11 11 | Hungria GlaxoSmithKline Biologicals SA Tel: + 36 80088309 |
Dinamarca GlaxoSmithKline Pharma A/S Tel: + 45 36 35 91 00 | Malta GlaxoSmithKline Biologicals SA Tel: + 356 80065004 |
Alemanha GlaxoSmithKline GmbH & Co. KG Tel: + 49 (0)89 360448701 | Países Baixos GlaxoSmithKline BV Tel: + 31 (0)33 2081100 |
Estônia GlaxoSmithKline Biologicals SA Tel: +372 8002640 | Noruega GlaxoSmithKline AS Tel: + 47 22 70 20 00 |
Grécia GlaxoSmithKline Μονοπρ?σωπη A.E.B.E. Tel: + 30 210 68 82 100 | Áustria GlaxoSmithKline Pharma GmbH. Tel: + 43 (0)1 970750 |
Espanha GlaxoSmithKline, S.A. Tel: + 34 900 202 700 | Polônia GSK Services Sp. z o.o. Tel: + 48 (22) 576 9000 |
França Laboratoire GlaxoSmithKline Tel: + 33 (0) 1 39 17 84 44 Croácia GlaxoSmithKline Biologicals SA Tel: + 385 800787089 | Portugal Smith Kline & French Portuguesa - Produtos Farmacêuticos, Lda. Tel: + 351 21 412 95 00 Romênia GlaxoSmithKline Biologicals SA Tel: +40 800672524 |
Irlanda GlaxoSmithKline (Irlanda) Ltd Tel: + 353 (0)1 495 5000 | Eslovênia GlaxoSmithKline Biologicals SA Tel: + 386 80688869 |
Islândia Vistor hf. Tel: +354 535 7000 | Eslováquia GlaxoSmithKline Biologicals SA Tel: + 421 800500589 |
Itália GlaxoSmithKline S.p.A. Tel: + 39 (0)45 774 1111 | Finlândia GlaxoSmithKline Oy Tel: + 358 10 30 30 30 |
Chipre GlaxoSmithKline Biologicals SA Tel: + 357 80070017 | Suécia GlaxoSmithKline AB Tel: + 46 (0)8 638 93 00 |
Letônia GlaxoSmithKline Biologicals SA Tel: + 371 80205045 | Reino Unido(Irlanda do Norte) GlaxoSmithKline Biologicals SA Tel: +44 (0)800 221 441 |
Data da última revisão deste prospecto:04/2023
Outras fontes de informação
A informação detalhada sobre este medicamento está disponível no site da Agência Europeia de Medicamentos http://www.ema.europa.eu, e no site da Agência Espanhola de Medicamentos e Produtos Sanitários (AEMPS) (http://www.aemps.gob.es/).
---------------------------------------------------------------------------------------------------------------------
Esta informação está destinada apenas a profissionais de saúde:
Durante o armazenamento pode ser observado um depósito fino de cor branca com uma camada translúcida e incolor por cima.
Deve-se ressuspender a vacina antes de seu uso. Uma vez ressuspendida, a vacina terá uma aparência branca, turva e uniforme.
Ressuspensão da vacina para obter uma suspensão branca, turva e uniforme
Deve-se ressuspender a vacina seguindo os passos que se indicam a seguir.
- Segurar a seringa com a boca para cima com a mão fechada.
- Agitar a seringa virando-a de cabeça para baixo e novamente de cabeça para cima.
- Repetir esta ação de forma vigorosa durante, pelo menos, 15 segundos.
- Inspecionar novamente a vacina:
- Se a vacina se mostra como uma suspensão branca, turva e uniforme, está pronta para ser usada (não deve ter uma aparência translúcida).
- Se a vacina ainda não se mostra como uma suspensão branca, turva e uniforme, vire-a de cabeça para baixo e novamente de cabeça para cima durante, pelo menos, outros 15 segundos e, em seguida, inspecione novamente.
Antes da administração, a vacina deve ser inspecionada visualmente para observar se tem alguma partícula estranha e/ou aparência física anormal. Em caso de observar alguma dessas circunstâncias, não administre a vacina.
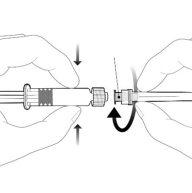
Instruções para a seringa pré-carregada após a ressuspensão
| Segure a seringa pelo corpo, não pelo émbolo. Desrosqueie a tampa da seringa girando-a no sentido contrário ao das agulhas do relógio. | |
| Para inserir a agulha, conecte a base ao adaptador luer-locke gire-o um quarto de volta no sentido das agulhas do relógio até que sinta que se bloqueia. Não retire o émbolo da seringa do corpo. Se isso ocorrer, não administre a vacina. |
Eliminação de resíduos
A eliminação do medicamento não utilizado e de todos os materiais que tenham estado em contato com ele será realizada de acordo com a regulamentação local.
- País de registo
- Substância ativa
- Requer receita médicaSim
- Fabricante
- Esta informação é apenas para referência e não constitui aconselhamento médico. Consulte sempre um médico antes de tomar qualquer medicamento. A Oladoctor não se responsabiliza por decisões médicas baseadas neste conteúdo.
- Alternativas a TWINRIX ADULTOS, SUSPENSÃO INJETÁVEL EM SERINGA PREENCHIDAForma farmacêutica: INJETÁVEL, DesconhecidaSubstância ativa: combinationsFabricante: Glaxosmithkline BiologicalsRequer receita médicaForma farmacêutica: INJETÁVEL, DesconhecidaSubstância ativa: combinationsFabricante: Glaxosmithkline BiologicalsRequer receita médicaForma farmacêutica: INJETÁVEL, DesconhecidaSubstância ativa: combinationsFabricante: Glaxosmithkline BiologicalsRequer receita médica
Alternativas a TWINRIX ADULTOS, SUSPENSÃO INJETÁVEL EM SERINGA PREENCHIDA noutros países
As melhores alternativas com o mesmo princípio ativo e efeito terapêutico.
Alternativa a TWINRIX ADULTOS, SUSPENSÃO INJETÁVEL EM SERINGA PREENCHIDA em Ukraine
Médicos online para TWINRIX ADULTOS, SUSPENSÃO INJETÁVEL EM SERINGA PREENCHIDA
Avaliação de posologia, efeitos secundários, interações, contraindicações e renovação da receita de TWINRIX ADULTOS, SUSPENSÃO INJETÁVEL EM SERINGA PREENCHIDA – sujeita a avaliação médica e regras locais.