Como usar TECVAYLI 10 mg/mL Solução Injetável
Introdução
Prospecto: informação para o paciente
TECVAYLI 10 mg/ml solução injetável
TECVAYLI 90 mg/ml solução injetável
teclistamab
Este medicamento está sujeito a acompanhamento adicional, o que agilizará a detecção de nova informação sobre sua segurança. Pode contribuir comunicando os efeitos adversos que possa ter. A parte final da seção 4 inclui informação sobre como comunicar esses efeitos adversos.
Leia todo o prospecto detenidamente antes de começar a receber este medicamento, porque contém informação importante para si.
- Conserva este prospecto, porque pode ter que voltar a lê-lo.
- Se tiver alguma dúvida, consulte o seu médico ou enfermeiro.
- Se experimentar efeitos adversos, consulte o seu médico ou enfermeiro, mesmo que se trate de efeitos adversos que não aparecem neste prospecto. Ver seção 4.
Conteúdo do prospecto
- O que é TECVAYLI e para que é utilizado
- O que precisa saber antes de começar a usar TECVAYLI
- Como usar TECVAYLI
- Possíveis efeitos adversos
- Conservação de TECVAYLI
- Conteúdo do envase e informação adicional
1. O que é TECVAYLI e para que é utilizado
TECVAYLI é um medicamento contra o cancro que contém o princípio ativo «teclistamab» e é utilizado para tratar pacientes adultos com um tipo de cancro de medula óssea denominado mieloma múltiplo.
É utilizado em pacientes que receberam pelo menos outras três classes de tratamento que ou não funcionaram ou deixaram de funcionar.
Como actua TECVAYLI
TECVAYLI é um anticorpo, um tipo de proteína que foi projetado para reconhecer alvos específicos do seu organismo e unir-se a eles. TECVAYLI dirige-se ao antígeno de maturação de linfócitos B (BCMA), o qual se encontra nas células cancerosas do mieloma múltiplo, e ao grupo de diferenciação 3 (CD3), o qual se encontra nas chamadas células T do seu sistema imunológico. Este medicamento actua unindo-se a essas células e agrupando-as, para que o seu sistema imunitário possa destruir as células cancerosas do mieloma múltiplo.
2. O que precisa saber antes de começar a usar TECVAYLI
Não use TECVAYLIse é alérgico a teclistamab ou a algum dos outros componentes deste medicamento (incluídos na seção 6).
Se não tem certeza se é alérgico, fale com o seu médico ou enfermeiro antes de usar TECVAYLI.
Advertências e precauções
Consulte o seu médico ou enfermeiro antes de começar a usar TECVAYLI se teve um acidente vascular cerebral ou convulsões nos últimos 6 meses.
TECVAYLI e as vacinas
Se foi vacinado recentemente ou se vai ser vacinado, consulte o seu médico ou enfermeiro antes de usar TECVAYLI.
Não deve ser administrada vacina elaborada com organismos vivos desde quatro semanas antes e até quatro semanas depois de ser tratado com TECVAYLI.
Provas e check-ups
Antes de começar a usar TECVAYLI,o seu médico reverá os seus hemogramas em busca de sinais de infecção. Se tiver alguma infecção, será tratado antes de começar TECVAYLI. O seu médico também verificará se está grávida ou em período de amamentação.
Durante o tratamento com TECVAYLI, o seu médico supervisionará os seus efeitos adversos. O seu médico verificará periodicamente os seus hemogramas, pois a cifra de células sanguíneas e outros componentes sanguíneos pode diminuir.
Esteja atento a possíveis efeitos adversos graves.
Informar imediatamente o seu médico ou enfermeiro se experimentar algum dos seguintes:
- Sinais de uma afecção conhecida como «síndrome de libertação de citocinas» (SLC). O síndrome de libertação de citocinas é uma reação imunitária grave com sintomas como febre, arrepios, náuseas, cefaleia, taquicardia, sensação de tontura e dificuldade para respirar.
- Efeitos sobre o sistema nervoso. Os sintomas incluem sensação de confusão, sensação de estar menos alerta, sonolência ou dificuldade para escrever e/ou falar. Alguns destes podem ser sinais de uma reação imunitária grave denominada «síndrome de neurotoxicidade associada a células imunoefectoras» (ICANS, por suas siglas em inglês).
- Sinais e sintomas de uma infecção.
Se observar algum dos sinais acima, informe o seu médico ou enfermeiro.
Crianças e adolescentes
Não administrar TECVAYLI a crianças ou adolescentes menores de 18 anos de idade, dado que se desconhece como este medicamento pode afetá-los.
Outros medicamentos e TECVAYLI
Informar o seu médico ou enfermeiro se está a tomar, tomou recentemente ou possa ter que tomar qualquer outro medicamento. Isso inclui os medicamentos que se podem obter sem receita e as plantas medicinais.
Gravidez e amamentação
Desconhece-se se TECVAYLI afeta o futuro bebê ou se passa para o leite materno.
Gravidez - informação para as mulheres
Se está grávida, acredita que possa estar grávida ou tem intenção de engravidar, consulte o seu médico ou enfermeiro antes de utilizar TECVAYLI.
Se engravidar enquanto está em tratamento com este medicamento, informe imediatamente o seu médico ou enfermeiro.
Gravidez - informação para os homens
Se a sua parceira engravidar enquanto está em tratamento com este medicamento, informe imediatamente o seu médico.
Anticoncepcionais
Se si ou a sua parceira pode engravidar, deve utilizar um método anticonceptivo eficaz durante o tratamento e durante os 3 meses seguintes após a interrupção do tratamento com TECVAYLI.
Amamentação
Si e o seu médico decidirão se o benefício da amamentação é maior que o risco para o seu bebê. Se si e o seu médico decidirem que deve deixar de utilizar este medicamento, não deve dar de mamar durante os 3 meses seguintes à interrupção do tratamento.
Condução e uso de máquinas
Algumas pessoas podem sentir-se cansadas, tontas ou confusas quando usam TECVAYLI. Não conduza, utilize ferramentas nem maneje maquinaria pesada nem realize atividades que possam supor um perigo para si até pelo menos 48 horas após receber a sua terceira dose de TECVAYLI, ou segundo o seu médico indicar.
TECVAYLI contém sódio
TECVAYLI contém menos de 1 mmol de sódio (23 mg) por dose; isto é, essencialmente «isento de sódio».
3. Como usar TECVAYLI
Quantidade administrada
O seu médico determinará a sua dose de TECVAYLI. A dose dependerá do seu peso corporal. As duas primeiras doses serão mais baixas.
TECVAYLI é administrado da seguinte forma:
- Serão administrados 0,06 mg por cada quilograma de peso corporal para a sua primeira dose.
- Serão administrados 0,3 mg por cada quilograma de peso corporal como a sua segunda dose 2-7 dias depois.
- Em seguida, receberá uma «dose de manutenção» de 1,5 mg por cada quilograma de peso corporal 2-7 dias depois da segunda dose.
- Posteriormente, seguirá a receber uma «dose de manutenção» uma vez por semana enquanto se beneficiar do uso de TECVAYLI.
O seu médico supervisionará os seus efeitos adversos após cada uma das três primeiras doses. Farão isso durante 2 dias após cada dose.
Deve permanecer perto de um centro sanitário após as três primeiras doses por si experimentar efeitos adversos.
Como é administrado o medicamento
TECVAYLI será administrado por um médico ou um enfermeiro em forma de injeção sob a sua pele (injeção «subcutânea»). É administrado na zona do estômago (abdomen) ou no muslo.
Outros medicamentos administrados durante o tratamento com TECVAYLI
Serão administrados medicamentos 1-3 horas antes de cada uma das três primeiras doses de TECVAYLI, que ajudam a reduzir a possibilidade de que se produzam efeitos adversos, tais como o síndrome de libertação de citocinas. Estes podem incluir:
- medicamentos para reduzir o risco de uma reação alérgica (antihistamínicos)
- medicamentos para reduzir o risco de inflamação (corticosteroides)
- medicamentos para reduzir o risco de febre (como paracetamol)
É possível que também sejam administrados estes medicamentos para doses posteriores de TECVAYLI em função dos sintomas que apresente.
Também é possível que lhe sejam administrados medicamentos adicionais em função dos sintomas que apresente ou da sua história clínica.
Se lhe for administrada mais TECVAYLI do que deve
Este medicamento será administrado pelo seu médico ou enfermeiro e é pouco provável que lhe seja administrada uma quantidade excessiva. Em caso de que lhe seja administrada uma quantidade excessiva (uma sobredose), o seu médico o examinará para ver se apresenta efeitos adversos.
Se esquecer a sua consulta para a administração de TECVAYLI
É muito importante comparecer a todas as suas consultas. Se não comparecer a uma consulta, concerte outra o mais rápido possível.
Se tiver alguma outra dúvida sobre o uso deste medicamento, pergunte ao seu médico ou enfermeiro.
4. Possíveis efeitos adversos
Como todos os medicamentos, este medicamento pode produzir efeitos adversos, embora nem todas as pessoas os sofram.
Efeitos adversos graves
Solicite atenção médica de imediato se experimentar algum dos seguintes efeitos adversos graves, que poderiam ser graves e até mortais.
Muito frequentes (podem afetar mais de 1 em cada 10 pessoas):
- reação imunitária grave («síndrome de libertação de citocinas») que pode provocar febre, arrepios, náuseas, cefaleia, taquicardia, sensação de tontura e dificuldade para respirar
- baixo nível de anticorpos chamados «imunoglobulinas» no sangue (hipogamaglobulinemia), o que pode tornar mais prováveis as infecções
- níveis baixos de um tipo de glóbulos brancos (neutropenia)
- infecção, que pode incluir febre, arrepios, tremores, tosse, dificuldade para respirar, respiração rápida e pulso rápido
Frequentes (podem afetar até 1 em cada 10 pessoas):
- Efeitos sobre o sistema nervoso. Estes podem ser sinais de uma reação imunitária grave denominada «síndrome de neurotoxicidade associada a células imunoefectoras» (ICANS). Alguns dos sintomas são:
- sensação de confusão
- sensação de estar menos alerta
- dificuldades para escrever
- dificuldades para falar
- sonolência
- perda da habilidade para realizar movimentos habilidosos e gestos (apesar de ter a habilidade física e o desejo de realizá-los
Informar imediatamente o seu médico se experimentar algum dos efeitos adversos graves acima mencionados.
Outros efeitos adversos
A seguir são enumerados outros efeitos adversos. Se experimentar algum destes efeitos adversos, informe o seu médico ou enfermeiro.
Muito frequentes (podem afetar mais de 1 em cada 10 pessoas):
- infecção pulmonar (pneumonia)
- infecção por COVID-19 causada por um vírus chamado coronavírus (SARS-CoV-2)
- infecção de nariz, seios ou garganta (infecção das vias respiratórias altas)
- níveis baixos de glóbulos vermelhos no sangue (anemia)
- níveis baixos de «plaquetas» no sangue (células que ajudam à coagulação do sangue; trombocitopenia)
- baixo número de glóbulos brancos no sangue (leucopenia)
- níveis baixos de um tipo de glóbulos brancos no sangue (linfopenia)
- nível baixo de «fosfato», «magnésio» ou «potássio» no sangue (hipofosfatemia, hipomagnesemia, hipopotasemia)
- aumento do nível de «cálcio» (hipercalcemia)
- aumento de «fosfatase alcalina» no sangue
- perda de apetite
- sensação de náuseas, diarreia, constipação, vômitos
- dor de cabeça
- dano nos nervos que pode causar formigamento, entorpecimento, dor ou perda da sensação de dor
- pressão arterial elevada (hipertensão)
- sangramento que pode ser grave (hemorragia)
- tosse
- falta de ar (dispnéia)
- febre
- sensação de cansaço intenso
- dor ou desconforto muscular
- inchaço de mãos, tornozelos ou pés (edema)
- reações cutâneas no local da injeção ou perto dele, incluindo vermelhidão da pele, coceira, inchaço, dor, hematomas, erupção, sangramento
Frequentes (podem afetar até 1 em cada 10 pessoas)
- infecção grave em todo o organismo (sepsis)
- infecção da pele que provoca vermelhidão (celulite)
- cifra baixa de um tipo de glóbulos brancos no sangue com febre (neutropenia febril)
- níveis baixos de «fibrinogênio», um tipo de proteína no sangue, tornando mais difícil a formação de coágulos
- alteração da função cerebral (encefalopatia)
- nível baixo de «cálcio» ou «sódio» no sangue (hipocalcemia ou hiponatremia)
- nível alto de «potássio» no sangue (hiperpotasemia)
- nível baixo de «albumina» no sangue (hipoalbuminemia)
- nível baixo de oxigênio no sangue (hipóxia)
- nível alto de «gama-glutamiltransferase» no sangue
- nível alto de enzimas hepáticas «transaminases» no sangue
- nível alto de «creatinina» no sangue
- nível alto de «amilase» no sangue (hiperamilasemia)
- nível alto de «lipase» no sangue (hiperlipasemia)
- as provas sanguíneas podem mostrar que leva mais tempo a coagulação do sangue (RIN aumentado e TTP prolongado)
Comunicação de efeitos adversos
Se experimentar algum tipo de efeito adverso, consulte o seu médico ou enfermeiro, mesmo que se trate de possíveis efeitos adversos que não aparecem neste prospecto. Também pode comunicá-los diretamente através do sistema nacional de notificação incluído no Apêndice V. Mediante a comunicação de efeitos adversos, pode contribuir para fornecer mais informação sobre a segurança deste medicamento.
5. Conservação de TECVAYLI
TECVAYLI será conservado pelo seu médico no hospital ou no centro médico.
Mantenha este medicamento fora da vista e do alcance das crianças.
Não utilize este medicamento após a data de validade que aparece na caixa e na etiqueta do frasco após «CAD». A data de validade é o último dia do mês que se indica.
Conservar em frigorífico (entre 2 ºC e 8 ºC). Não congelar.
Conservar no embalagem original para protegê-lo da luz.
Os medicamentos não devem ser jogados fora pelos esgotos nem para o lixo. O seu profissional de saúde descartará os medicamentos que já não se utilizam. Desta forma, ajudará a proteger o meio ambiente.
6. Conteúdo do envase e informação adicional
Composição de TECVAYLI
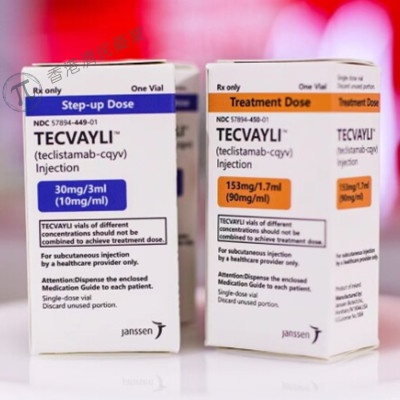
- O princípio ativo é teclistamab. TECVAYLI apresenta-se em duas concentrações diferentes:
- 10 mg/ml – um frasco de 3 ml contém 30 mg de teclistamab
- 90 mg/ml – um frasco de 1,7 ml contém 153 mg de teclistamab
- Os outros componentes são dihidratado de EDTA sódico, ácido acético glacial, polissorbato 20, trihidrato de acetato de sódio, sacarose, água para preparações injetáveis (ver «TECVAYLI contém sódio» na seção 2).
Aspecto de TECVAYLI e conteúdo do envase
TECVAYLI é uma solução injetável (injetável) líquida incolor a amarelo claro.
TECVAYLI apresenta-se em um envase de cartão que contém 1 frasco de vidro.
Título da autorização de comercialização
Janssen-Cilag International NV
Turnhoutseweg 30
B-2340 Beerse
Bélgica
Responsável pela fabricação
Janssen Biologics B.V.
Einsteinweg 101
2333 CB Leiden
Países Baixos
Podem solicitar mais informações sobre este medicamento dirigindo-se ao representante local do título da autorização de comercialização:
Bélgica Janssen-Cilag NV Tel: +32 14 64 94 11 | Lituânia UAB "JOHNSON & JOHNSON" Tel: +370 5 278 68 88 |
| Luxemburgo Janssen-Cilag NV Tel: +32 14 64 94 11 |
República Checa Janssen-Cilag s.r.o. Tel: +420 227 012 227 | Hungria Janssen-Cilag Kft. Tel: +36 1 884 2858 |
Dinamarca Janssen-Cilag A/S Tel: +45 4594 8282 | Malta AM MANGION LTD Tel: +356 2397 6000 |
Alemanha Janssen-Cilag GmbH Tel: +49 2137 955 955 | Países Baixos Janssen-Cilag B.V. Tel: +31 76 711 1111 |
Estônia UAB "JOHNSON & JOHNSON" filial na Estônia Tel: +372 617 7410 | Noruega Janssen-Cilag AS Tel: +47 24 12 65 00 |
Grécia Janssen-Cilag Φαρμακευτικ? Α.Ε.Β.Ε. Tel: +30 210 80 90 000 | Áustria Janssen-Cilag Pharma GmbH Tel: +43 1 610 300 |
Espanha Janssen-Cilag, S.A. Tel: +34 91 722 81 00 | Polônia Janssen-Cilag Polska Sp. z o.o. Tel: +48 22 237 60 00 |
França Janssen-Cilag Tel: 0 800 25 50 75 / +33 1 55 00 40 03 | Portugal Janssen-Cilag Farmacêutica, Lda. Tel: +351 214 368 600 |
Croácia Johnson & Johnson S.E. d.o.o. Tel: +385 1 6610 700 | Romênia Johnson & Johnson România SRL Tel: +40 21 207 1800 |
Irlanda Janssen Sciences Ireland UC Tel: +353 1 800 709 122 | Eslovênia Johnson & Johnson d.o.o. Tel: +386 1 401 18 00 |
Islândia Janssen-Cilag AB c/o Vistor hf. Tel: +354 535 7000 | República Eslovaca Johnson & Johnson, s.r.o. Tel: +421 232 408 400 |
Itália Janssen-Cilag SpA Tel: 800.688.777 / +39 02 2510 1 | Finlândia Janssen-Cilag Oy Tel: +358 207 531 300 |
| Suécia Janssen-Cilag AB Tel: +46 8 626 50 00 |
Letônia UAB "JOHNSON & JOHNSON" filial na Letônia Tel: +371 678 93561 | Reino Unido (Irlanda do Norte) Janssen Sciences Ireland UC Tel: +44 1 494 567 444 |
Data da última revisão deste prospecto:
Este medicamento foi autorizado com uma «autorização condicional». Esta modalidade de autorização significa que se espera obter mais informações sobre este medicamento.
A Agência Europeia de Medicamentos reverá as informações novas sobre este medicamento pelo menos uma vez por ano e este prospecto será atualizado quando necessário.
Outras fontes de informação
A informação detalhada sobre este medicamento está disponível no site da Agência Europeia de Medicamentos: http://www.ema.europa.eu.
No site da Agência Europeia de Medicamentos, pode encontrar este prospecto em todas as línguas da União Europeia/Espaço Econômico Europeu.
Esta informação é destinada apenas a profissionais de saúde:
É muito importante seguir estritamente as instruções de preparação e administração fornecidas nesta seção para reduzir ao mínimo possíveis erros de dosagem com os frascos de TECVAYLI 10 mg/ml e TECVAYLI 90 mg/ml.
TECVAYLI deve ser administrado apenas por injeção subcutânea. Não administrar TECVAYLI por via intravenosa.
TECVAYLI deve ser administrado por um profissional de saúde com pessoal médico adequadamente treinado e com o equipamento médico apropriado para o manejo de reações graves, incluindo o síndrome de libertação de citocinas.
Os frascos de TECVAYLI 10 mg/ml e TECVAYLI 90 mg/ml são de uso único.
Não se devem combinar frascos de TECVAYLI de diferentes concentrações para obter a dose de manutenção.
Para preparar e administrar TECVAYLI, deve utilizar-se uma técnica asséptica.
A eliminação do medicamento não utilizado e de todos os materiais que tenham estado em contacto com ele será realizada de acordo com a regulamentação local.
Preparação de TECVAYLI
- Verifique a dose prescrita de cada injeção de TECVAYLI. Para reduzir ao mínimo os erros, utilize as seguintes tabelas para preparar a injeção de TECVAYLI.
- Utilize a Tabela 1 para determinar a dose total, o volume de injeção e o número de frascos necessários com base no peso corporal atual do paciente para a escalada de dose 1 utilizando o frasco de TECVAYLI 10 mg/ml.
Tabela 1: Volumes de injeção de TECVAYLI (10 mg/ml) para a escalada de dose 1 (0,06 mg/kg)
Escalada de dose 1 (0,06 mg/kg) | Peso corporal (kg) | Dose total (mg) | Volume de injeção (ml) | Número de frascos (1 frasco = 3 ml) |
35-39 | 2,2 | 0,22 | 1 | |
40-44 | 2,5 | 0,25 | 1 | |
45-49 | 2,8 | 0,28 | 1 | |
50-59 | 3,3 | 0,33 | 1 | |
60-69 | 3,9 | 0,39 | 1 | |
70-79 | 4,5 | 0,45 | 1 | |
80-89 | 5,1 | 0,51 | 1 | |
90-99 | 5,7 | 0,57 | 1 | |
100-109 | 6,3 | 0,63 | 1 | |
110-119 | 6,9 | 0,69 | 1 | |
120-129 | 7,5 | 0,75 | 1 | |
130-139 | 8,1 | 0,81 | 1 | |
140-149 | 8,7 | 0,87 | 1 | |
150-160 | 9,3 | 0,93 | 1 |
- Utilize a Tabela 2 para determinar a dose total, o volume de injeção e o número de frascos necessários com base no peso corporal atual do paciente para a escalada de dose 2 utilizando o frasco de TECVAYLI 10 mg/ml.
Tabela 2: Volumes de injeção de TECVAYLI (10 mg/ml) para a escalada de dose 2 (0,3 mg/kg)
Escalada de dose 2 (0,3 mg/kg) | Peso corporal (kg) | Dose total (mg) | Volume de injeção (ml) | Número de frascos (1 frasco = 3 ml) |
35-39 | 11 | 1,1 | 1 | |
40-44 | 13 | 1,3 | 1 | |
45-49 | 14 | 1,4 | 1 | |
50-59 | 16 | 1,6 | 1 | |
60-69 | 19 | 1,9 | 1 | |
70-79 | 22 | 2,2 | 1 | |
80-89 | 25 | 2,5 | 1 | |
90-99 | 28 | 2,8 | 1 | |
100-109 | 31 | 3,1 | 2 | |
110-119 | 34 | 3,4 | 2 | |
120-129 | 37 | 3,7 | 2 | |
130-139 | 40 | 4,0 | 2 | |
140-149 | 43 | 4,3 | 2 | |
150-160 | 47 | 4,7 | 2 |
- Utilize a Tabela 3 para determinar a dose total, o volume de injeção e o número de frascos necessários com base no peso corporal atual do paciente para a dose de manutenção utilizando o frasco de TECVAYLI 90 mg/ml.
Tabela 3: Volumes de injeção de TECVAYLI (90 mg/ml) para a dose de manutenção (1,5 mg/kg)
Dose de manutenção (1,5 mg/kg) | Peso corporal (kg) | Dose total (mg) | Volume de injeção (ml) | Número de frascos (1 frasco = 7 ml) |
35-39 | 56 | 0,62 | 1 | |
40-44 | 63 | 0,70 | 1 | |
45-49 | 70 | 0,78 | 1 | |
50-59 | 82 | 0,91 | 1 | |
60-69 | 99 | 1,1 | 1 | |
70-79 | 108 | 1,2 | 1 | |
80-89 | 126 | 1,4 | 1 | |
90-99 | 144 | 1,6 | 1 | |
100-109 | 153 | 1,7 | 1 | |
110-119 | 171 | 1,9 | 2 | |
120-129 | 189 | 2,1 | 2 | |
130-139 | 198 | 2,2 | 2 | |
140-149 | 216 | 2,4 | 2 | |
150-160 | 234 | 2,6 | 2 |
- Retire o frasco de TECVAYLI da concentração correspondente do armazenamento refrigerado (2 ºC - 8 ºC) e equilibre-o à temperatura ambiente (15 ºC - 30 ºC), se necessário, durante pelo menos 15 minutos. Não aqueça TECVAYLI de nenhuma outra forma.
- Uma vez equilibrado, dê voltas suaves ao frasco durante aproximadamente 10 segundos para misturá-lo. Não agite.
- Extraia o volume de injeção necessário de TECVAYLI do (dos) frasco(s) em uma seringa de tamanho apropriado usando uma agulha de transferência.
- Cada volume de injeção não deve exceder 2,0 ml. Divida as doses que necessitam de mais de 2,0 ml de forma equitativa em várias seringas.
- TECVAYLI é compatível com agulhas de aço inoxidável e material de seringas de polipropileno e policarbonato.
- Substitua a agulha de transferência por outra de tamanho apropriado para a injeção.
- Inspeccione TECVAYLI visualmente em busca de partículas e decoloração antes da administração. Não o utilize se a solução estiver descolorida, ou turva, ou se houver partículas estranhas.
- A solução injetável de TECVAYLI é incolor a amarelo claro.
Administração de TECVAYLI
- Injete o volume necessário de TECVAYLI no tecido subcutâneo do abdômen (local de injeção preferido). Alternativamente, TECVAYLI pode ser injetado no tecido subcutâneo da coxa. Se forem necessárias várias injeções, os locais de injeção de TECVAYLI devem estar separados por pelo menos 2 cm.
- Não injete em tatuagens ou cicatrizes ou em zonas em que a pele esteja enrubescida, magoada, sensível, dura ou não intacta.
Rastreabilidade
Com o objetivo de melhorar a rastreabilidade dos medicamentos biológicos, o nome e o número de lote do medicamento administrado devem estar claramente registrados.
- País de registo
- Substância ativa
- Requer receita médicaSim
- Fabricante
- Esta informação é apenas para referência e não constitui aconselhamento médico. Consulte sempre um médico antes de tomar qualquer medicamento. A Oladoctor não se responsabiliza por decisões médicas baseadas neste conteúdo.
- Alternativas a TECVAYLI 10 mg/mL Solução InjetávelForma farmacêutica: INJETÁVEL, 90 mg/mlSubstância ativa: teclistamabFabricante: Janssen-Cilag International N.VRequer receita médicaForma farmacêutica: PERFURAÇÃO INJETÁVEL, 50 mgSubstância ativa: brentuximab vedotinFabricante: Takeda Pharma A/SRequer receita médicaForma farmacêutica: PERFURAÇÃO INJETÁVEL, 100 mgSubstância ativa: belantamab mafodotinFabricante: Glaxosmithkline Trading Services LimitedRequer receita médica
Médicos online para TECVAYLI 10 mg/mL Solução Injetável
Avaliação de posologia, efeitos secundários, interações, contraindicações e renovação da receita de TECVAYLI 10 mg/mL Solução Injetável – sujeita a avaliação médica e regras locais.