SANDOSTATIN LAR 30 mg PÓ E SOLVENTE PARA SUSPENSÃO INJETÁVEL


Como usar SANDOSTATIN LAR 30 mg PÓ E SOLVENTE PARA SUSPENSÃO INJETÁVEL
Introdução
Prospecto: informação para o paciente
SANDOSTATIN LAR 30 mg pó e dissolvente para suspensão injetável
octreotida
Leia todo o prospecto atentamente antes de começar a usar este medicamento, pois contém informações importantes para si.
- Conserva este prospecto, pois pode ter que relê-lo.
- Se tiver alguma dúvida, consulte o seu médico, farmacêutico ou enfermeiro.
- Este medicamento foi prescrito apenas para si, e não deve dá-lo a outras pessoas, mesmo que tenham os mesmos sintomas que si, pois pode prejudicá-las.
- Se experimentar efeitos adversos, consulte o seu médico, farmacêutico ou enfermeiro, mesmo que se trate de efeitos adversos que não aparecem neste prospecto. Ver seção 4.
Conteúdo do prospecto
- O que é Sandostatin LAR e para que é utilizado
- O que precisa saber antes de começar a usar Sandostatin LAR
- Como usar Sandostatin LAR
- Possíveis efeitos adversos
- Conservação de Sandostatin LAR
- Conteúdo do envase e informação adicional
1. O que é Sandostatin LAR e para que é utilizado
Sandostatin LAR é um composto sintético derivado da somatostatina. A somatostatina encontra-se normalmente no corpo humano, onde inibe a libertação de algumas hormonas, como a hormona do crescimento. As vantagens de Sandostatin LAR em relação à somatostatina são que é mais potente e os seus efeitos são mais duradouros.
Sandostatin LAR é utilizado
- para tratar a acromegalia,
A acromegalia é uma doença em que o corpo produz demasiada hormona do crescimento. Normalmente, a hormona do crescimento controla o crescimento dos tecidos, órgãos e ossos. Um excesso de hormona do crescimento supõe um aumento no tamanho dos ossos e tecidos, especialmente nas mãos e pés. Sandostatin LAR reduz notavelmente os sintomas da acromegalia, que incluem dor de cabeça, excesso de suor, formigamento nas mãos e pés, cansaço e dor nas articulações. Na maioria dos casos, a sobreprodução de hormona do crescimento é causada por aumento do tamanho da glândula pituitária (adenoma pituitário); o tratamento com Sandostatin LAR pode reduzir o tamanho do adenoma.
Sandostatin LAR é utilizado para tratar pessoas que sofrem de acromegalia:
- quando outros tipos de tratamento para a acromegalia (cirurgia ou radioterapia) não são adequados ou não funcionaram corretamente;
- após a radioterapia, para cobrir o período de tempo intermediário até que a radioterapia seja completamente eficaz.
- para aliviar os sintomas associados à sobreprodução de algumas hormonas específicas e outras substâncias relacionadas no estômago, intestino ou pâncreas.
A sobreprodução de algumas hormonas específicas e de outras substâncias naturais relacionadas pode ser causada por algumas alterações raras do estômago, intestino ou pâncreas. Isso altera o equilíbrio hormonal natural e provoca uma série de sintomas, como sofocos, diarreia, baixa tensão arterial, erupção cutânea e perda de peso. O tratamento com Sandostatin LAR ajuda a controlar esses sintomas.
- para tratar tumores neuroendócrinos localizados no intestino (p. ex., apêndice, intestino delgado ou cólon)
Os tumores neuroendócrinos são tumores raros que podem ser encontrados em diferentes partes do corpo. Sandostatin LAR também é utilizado para controlar o crescimento desses tumores, quando estão localizados no intestino (p. ex., apêndice, intestino delgado ou cólon)
- para tratar tumores pituitários que produzem demasiada hormona estimulante da tiróide (TSH).
Um excesso de hormona estimulante da tiróide (TSH) provoca hipertireoidismo. Sandostatin LAR é utilizado para tratar pessoas com tumores pituitários que produzem demasiada hormona estimulante da tiróide (TSH):
- quando outros tipos de tratamento (cirurgia ou radioterapia) não são adequados ou não funcionaram;
após a radioterapia, para cobrir o período até que a radioterapia seja completamente eficaz.
2. O que precisa saber antes de começar a usar Sandostatin LAR
Siga todas as instruções que o seu médico lhe der cuidadosamente. Podem ser diferentes da informação contida neste prospecto.
Leia as seguintes indicações antes de utilizar Sandostatin LAR.
Não use Sandostatin LAR:
- se é alérgico a octreotida ou a qualquer um dos outros componentes deste medicamento (incluídos na seção 6).
Advertências e precauções
Consulte o seu médico antes de começar a usar Sandostatin LAR:
- se sabe que tem atualmente cálculos na vesícula biliar, ou os teve no passado ou apresenta alguma complicação, como febre, calafrios, dor abdominal, ou coloração amarelada da pele ou dos olhos; informe o seu médico, pois o uso prolongado de Sandostatin LAR pode provocar a formação de cálculos biliares. O seu médico pode querer controlar a sua vesícula biliar periodicamente.
- se sabe que tem diabetes, pois Sandostatin LAR pode afetar os níveis de açúcar no sangue. Se é diabético, deve controlar regularmente os níveis de açúcar.
- se tem antecedentes de deficiência de vitamina B12; o seu médico pode controlar o nível de B12 periodicamente.
Análises e controles
Se receber tratamento com Sandostatin LAR durante um período prolongado de tempo, o seu médico pode controlar a sua função tireoidiana periodicamente.
O seu médico controlará a função do seu fígado.
O seu médico pode verificar o funcionamento das suas enzimas pancreáticas.
Crianças
Existe pouca experiência com o uso de Sandostatin LAR em crianças.
Uso de Sandostatin LAR com outros medicamentos
Comunique ao seu médico ou farmacêutico se está tomando, tomou recentemente ou pode ter que tomar qualquer outro medicamento.
Normalmente, pode continuar tomando outros medicamentos enquanto estiver em tratamento com Sandostatin LAR. No entanto, foram notificados que alguns medicamentos, como cimetidina, ciclosporina, bromocriptina, quinidina e terfenadina, são afetados por Sandostatin LAR.
Se está tomando um medicamento para controlar a pressão arterial (p. ex., um beta-bloqueador ou um antagonista dos canais de cálcio) ou um agente para controlar o equilíbrio de líquidos e eletrólitos, o seu médico pode precisar ajustar a dose.
Se é diabético, o seu médico pode precisar ajustar a dose de insulina.
Se vai receber tratamento com lutécio (177Lu) oxodotreotida, um radiofármaco, o seu médico pode interromper e/ou adaptar o tratamento com Sandostatin LAR durante um período curto de tempo.
Gravidez e lactação
Se está grávida ou em período de lactação, acredita que possa estar grávida ou tem intenção de engravidar, consulte o seu médico antes de utilizar este medicamento.
Somente se deve utilizar Sandostatin LAR durante a gravidez se for estritamente necessário.
As mulheres em idade fértil devem utilizar um método anticonceptivo eficaz durante o tratamento.
Não deve amamentar durante o tratamento com Sandostatin LAR. Desconhece-se se Sandostatin LAR passa para o leite materno.
Condução e uso de máquinas
Sandostatin LAR não tem efeitos ou estes são insignificantes sobre a capacidade de conduzir ou utilizar máquinas. No entanto, alguns desses efeitos adversos que pode sofrer durante o tratamento com Sandostatin LAR, como dor de cabeça e cansaço, podem reduzir a sua capacidade para conduzir e utilizar máquinas de forma segura.
Sandostatin contém sódio
Este medicamento contém menos de 1 mmol de sódio (23 mg) por frasco; isto é, é essencialmente “isento de sódio”.
3. Como usar Sandostatin LAR
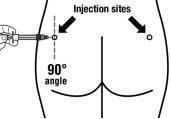
Sandostatin LAR deve ser administrado sempre como uma injeção no músculo dos glúteos. Com a administração repetida, deve ser utilizado o glúteo direito e esquerdo alternativamente.
Se usar mais Sandostatin LAR do que deve
Não foram notificadas reações adversas que supõem uma ameaça vital após uma sobredose com Sandostatin LAR.
Os sintomas de sobredose são: sofocos, micções frequentes, cansaço, depressão, ansiedade e falta de concentração.
Se achar que sofreu uma sobredose e apresenta algum desses sintomas, informe o seu médico imediatamente. Também pode ligar para o Serviço de Informação Toxicológica, Tel. 91 562 0420.
Se esquecer de usar Sandostatin LAR
Se esqueceu da sua injeção, recomenda-se que a mesma seja administrada assim que se lembrar, e depois continuar com a pauta habitual. Não vai provocar-lhe nenhum dano o facto de receber uma dose alguns dias mais tarde, mas podem reaparecer temporariamente os sintomas até que volte à pauta habitual de tratamento.
Se interromper o tratamento com Sandostatin LAR
Se interromper o tratamento com Sandostatin LAR, podem reaparecer os seus sintomas. Por isso, não interrompa o tratamento com Sandostatin LAR a menos que o seu médico o indique.
Se tiver alguma outra dúvida sobre o uso deste medicamento, pergunte ao seu médico, enfermeiro ou farmacêutico.
4. Possíveis efeitos adversos
Como todos os medicamentos, este medicamento pode produzir efeitos adversos, embora nem todas as pessoas os sofram.
Algumas reações adversas podem ser graves. Informe o seu médico imediatamente se sofrer algum dos seguintes:
Muito frequentes(podem afetar mais de 1 em cada 10 pessoas):
- Cálculos biliares, que conlevam dor de costas repentina.
- Demais açúcar no sangue.
Frequentes(podem afetar até 1 em cada 10 pessoas):
- Diminuição da atividade da glândula tireoidiana (hipotireoidismo) que causa alterações no ritmo do coração, no apetite ou no peso; cansaço, sensação de frio, ou inchaço na parte da frente do pescoço.
- Alterações nos análises da função tireoidiana.
- Inflamação da vesícula biliar (colecistite); os sintomas podem incluir dor na parte superior direita do abdômen, febre, náuseas, coloração amarelada da pele e dos olhos (icterícia).
- Muito pouco açúcar no sangue.
- Alteração da tolerância à glicose.
- Latido do coração lento.
Pouco frequentes(podem afetar até 1 em cada 100 pessoas):
- Sede, baixa eliminação de urina, cor escura na urina, pele seca e vermelha.
- Latido do coração rápido.
Outras reações adversas graves
- Reações de hipersensibilidade (alérgicas) incluindo urticária na pele.
- Um tipo de reação alérgica (anafilaxia) que pode causar dificuldade para engolir ou respirar, inchaço e formigamento, possivelmente com uma diminuição da tensão arterial com tontura ou perda de consciência.
- Uma inflamação da glândula do pâncreas (pancreatite); os sintomas podem incluir dor repentina na parte superior do abdômen, náuseas, vómitos, diarreia.
- Inflamação do fígado (hepatite); sintomas que podem incluir coloração amarelada da pele e dos olhos (icterícia), náuseas, vómitos, perda de apetite, sensação geral de mal-estar, coceira, urina ligeiramente colorida.
- Latido do coração irregular.
- Nível baixo no recuento de plaquetas no sangue; isto pode supor um aumento no sangramento ou na aparência de hematomas.
Informe o seu médico imediatamente se notar algum dos efeitos adversos anteriores.
Outros efeitos adversos:
Informe o seu médico, farmacêutico ou enfermeiro se notar algum dos efeitos adversos enumerados a seguir. Normalmente, são leves e tendem a desaparecer à medida que o tratamento avança.
Muito frequentes(podem afetar mais de 1 em cada 10 pessoas):
- Diarreia.
- Dor abdominal.
- Náuseas.
- Prisão de ventre.
- Flatulência (gases).
- Dor de cabeça.
- Dor local no lugar da injeção.
Frequentes(podem afetar até 1 em cada 10 pessoas):
- Desconforto no estômago após comer (dispepsia).
- Vómitos.
- Sensação de ter o estômago cheio.
- Fezes gordurosas.
- Fezes líquidas.
- Mudança de cor das fezes.
- Tontura.
- Perda de apetite.
- Alterações nos análises da função do fígado.
- Perda de cabelo.
- Dificuldade para respirar.
- Fraqueza.
Se experimentar qualquer tipo de efeito adverso, consulte o seu médico, enfermeiro ou farmacêutico.
Comunicação de efeitos adversos
Se experimentar qualquer tipo de efeito adverso, consulte o seu médico ou farmacêutico, mesmo que se trate de possíveis efeitos adversos que não aparecem neste prospecto. Também pode comunicá-los diretamente através do Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: http://www.notificaram.es. Mediante a comunicação de efeitos adversos, você pode contribuir para proporcionar mais informações sobre a segurança deste medicamento.
5. Conservação de Sandostatin LAR
Mantenha este medicamento fora da vista e do alcance das crianças.
Conserva este medicamento no embalagem original para protegê-lo da luz.
Conserva em frigorífico (entre 2°C e 8°C). Não congele.
Sandostatin LAR pode ser conservado por debaixo de 25°C no dia da injeção.
Não se deve conservar Sandostatin LAR após a reconstituição (deve ser utilizado imediatamente).
Não utilize este medicamento após a data de validade que aparece no envase após CAD. A data de validade é o último dia do mês que se indica.
Não utilize este medicamento se observar presença de partículas ou um cambio de cor.
Os medicamentos não devem ser jogados nos deságues ou na lixeira. Deposite os envases e os medicamentos que não precisa no ponto SIGRE da farmácia. Em caso de dúvida, pergunte ao seu farmacêutico como se livrar dos envases e dos medicamentos que não precisa. Dessa forma, ajudará a proteger o meio ambiente.
6. Conteúdo do envase e informação adicional
Composição de Sandostatin LAR
- O princípio ativo é octreotida.
Um frasco contém 30 mg de octreotida (como octreotida acetato).
- Os outros componentes do frasco são:
No pó (frasco): poli (DL-láctido-co-glicólido), manitol (E421).
No diluente (seringa pré-carregada): carboximetilcelulosa sódica, manitol (E421), poloxamer 188, água para preparações injetáveis
Aspecto de Sandostatin LAR e conteúdo do envase
Envases unitários que contêm um frasco de vidro de 6 ml com tampa de borracha (bromobutilo), selado com uma lingueta de alumínio, que contêm o pó para suspensão injetável e uma seringa pré-carregada de vidro incolor de 3 ml com um tampo frontal e um tampo de êmbolo (borracha clorobutilo) com 2 ml de diluente, envasados conjuntamente em uma bandeja blister selada com um adaptador ao frasco e uma agulha de injeção de segurança.
Envases múltiplos de três envases unitários, que cada um contém: um frasco de vidro de 6 ml com tampa de borracha (bromobutilo), selado com uma lingueta de alumínio, que contém o pó para suspensão injetável e uma seringa pré-carregada de vidro incolor de 3 ml com um tampo frontal e um tampo de êmbolo (borracha clorobutilo) com 2 ml de diluente, envasados conjuntamente em uma bandeja blister selada com um adaptador ao frasco e uma agulha de injeção de segurança.
Pode ser que apenas alguns tamanhos de envases sejam comercializados.
Título da autorização de comercialização
Novartis Farmacêutica, S.A.
Gran Vía de les Corts Catalanes, 764
08013 Barcelona
Responsável pela fabricação
Novartis Farmacêutica, S.A.
Gran Vía de les Corts Catalanes, 764
08013 Barcelona
Novartis Pharma GmbH
Jakov-Lind-Straße 5, Top 3.05 1020 Wien Áustria
Novartis Pharma NV
Medialaan 40/Bus 1, 1800 Vilvoorde, (Bélgica).
Novartis Healthcare A/S
Edvard Thomsens vej 14, DK-2300 Copenhagen S, (Dinamarca).
Novartis Finland Oy
Metsänneidonkuja 10, 02130 Espoo, (Finlândia)
Novartis Pharma SAS
8-10 rue Henri Sainte-Claire Deville, 92500 Rueil-Malmaison (França)
Novartis Pharma GmbH
Roonstrasse 25, 90429 Nürnberg, (Alemanha).
Novartis Pharma GmbH
Sophie-Germain-Strasse 10, 90443 Nürnberg (Alemanha).
Novartis Farma-Produtos Farmacêuticos, SA
Avenida Professor Doutor Cavaco Silva nº10E, Taguspark, 2740-255 Porto Salvo, (Portugal).
Novartis Farma S.p.A.
Via Provinciale Schito 131, 80058 Torre Annunziata, NA , (Itália).
Novartis Farma S.p.A.
Viale Luigi Sturzo 43, 20154 - Milan (MI) (Itália)
Novartis Sverige AB
Torshamnsgatan 48, 164 40 Kista (Suécia)
Novartis (Hellas) S.A.
12th km National Road Athinon-Lamia 14451 Metamorphosis Attiki, (Grécia).
Novartis Pharma B.V.
Haaksbergweg 16, 1101 BX Amsterdam (Holanda).
Novartis Poland Sp. z o.o.
15 Marynarska Street, 02-674 Varsovia (Polônia).
Novartis Hungária Kft.
Vasút u.13, 2040 Budaörs (Hungria).
Abbot Biologicals B.V.
Veerweg, 12. 8121 AA Olst (Holanda)
Este medicamento está autorizado nos estados membros do Espaço Económico Europeu com os seguintes nomes:
Áustria, Bulgária, Croácia, Chipre, República Checa, Dinamarca, Estônia, Finlândia, Alemanha, Grécia, Hungria, Islândia, Irlanda, Letônia, Lituânia, Malta, Noruega, Polônia, Romênia, Eslováquia, Eslovênia, Espanha, Suécia | Sandostatin LAR |
Bélgica, Luxemburgo, Holanda | Sandostatine LAR |
Itália, Portugal | Sandostatina LAR |
França | Sandostatine L.P. |
Data da última revisão deste prospecto:12/2023
Outras fontes de informação
A informação detalhada e atualizada deste medicamento está disponível na página Web da Agência Espanhola de Medicamentos e Produtos Sanitários (AEMPS) http://www.aemps.gob.es/
Esta informação está destinada apenas a profissionais do setor sanitário:
Quantidade de Sandostatin LAR que deve ser utilizada
Acromegalia
Recomenda-se iniciar o tratamento com a administração de 20 mg de Sandostatin LAR a intervalos de 4 semanas durante 3 meses. Os pacientes em tratamento com Sandostatin s.c. podem iniciar o tratamento com Sandostatin LAR no dia seguinte à última dose de Sandostatin s.c.. O ajuste de dose subsequente deve basear-se nas concentrações séricas da hormona do crescimento (GH) e do fator de crescimento 1 de tipo insulina/somatomedina C (IGF-1) e nos sintomas clínicos.
Para os pacientes nos quais os sintomas clínicos e os parâmetros bioquímicos (GH; IGF-1) não estão completamente controlados em um período de 3 meses (concentrações de GH ainda acima de 2,5 microgramas/L), pode-se aumentar a dose para 30 mg cada 4 semanas. Se, após 3 meses, os GH, IGF-1 e/ou os sintomas não estiverem adequadamente controlados na dose de 30 mg, a dose pode ser aumentada para 40 mg cada 4 semanas.
Para pacientes com concentrações de GH que se encontram constantemente abaixo de 1 micrograma/L, e com concentrações séricas de IGF-1 normalizadas, e nos quais a maioria dos sinais/sintomas reversíveis de acromegalia desapareceu após 3 meses de tratamento com 20 mg, podem ser administrados 10 mg de Sandostatin LAR cada 4 semanas. No entanto, especialmente neste grupo de pacientes, recomenda-se controlar estreitamente as concentrações séricas de GH e IGF-1, e os sinais/sintomas a esta dose baixa de Sandostatin LAR.
Para pacientes que estão sendo tratados com uma dose estabelecida de Sandostatin LAR, a avaliação de GH e IGF-1 deve ser realizada cada 6 meses.
Tumores endócrinos gastroenteropancreáticos
- Tratamento de pacientes com sintomas associados a tumores neuroendócrinos gastroenteropancreáticos funcionais
Recomenda-se iniciar o tratamento com a administração de 20 mg de Sandostatin LAR a intervalos de 4 semanas. Os pacientes em tratamento com Sandostatin s.c. devem continuar a dose previamente eficaz durante 2 semanas após a primeira injeção de Sandostatin LAR.
Para pacientes nos quais os sintomas e os marcadores biológicos estão bem controlados após 3 meses de tratamento, pode-se reduzir a dose para 10 mg de Sandostatin LAR cada 4 semanas.
Para pacientes nos quais os sintomas estão apenas parcialmente controlados após 3 meses de tratamento, pode-se aumentar a dose para 30 mg de Sandostatin LAR cada 4 semanas.
Para os dias nos quais podem aumentar os sintomas associados a tumores gastroenteropancreáticos durante o tratamento com Sandostatin LAR, recomenda-se a administração adicional de Sandostatin s.c. na dose utilizada antes do tratamento com Sandostatin LAR. Isso pode ocorrer principalmente nos 2 primeiros meses de tratamento até que se atinjam as concentrações terapêuticas de octreotida.
- Tratamento de pacientes com Tumores Neuroendócrinos avançados do intestino ou de origem desconhecida onde se excluíram os lugares de origem que não sejam do intestino
A dose recomendada de Sandostatin LAR é de 30 mg administrada cada 4 semanas. O tratamento com Sandostatin LAR para controlar o tumor deve ser continuado na ausência de progressão do tumor.
Tratamento de adenomas secretórios de TSH
O tratamento com Sandostatin LAR deve ser iniciado na dose de 20 mg a intervalos de 4 semanas durante 3 meses antes de considerar um ajuste de dose. Depois, a dose é ajustada com base na TSH e na resposta da hormona tireoidiana.
Instruções para a preparação e a injeção intramuscular de Sandostatin LAR.
SÓ PARA INJEÇÃO INTRAMUSCULAR
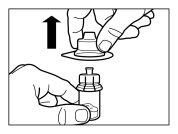
Componentes do kit de injeção:
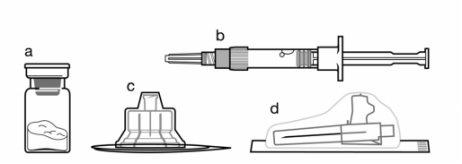
- Um frasco que contém o pó de Sandostatin LAR
- Uma seringa pré-carregada que contém o diluente para a reconstituição
- Um adaptador ao frasco para a reconstituição do medicamento
- Uma agulha de injeção de segurança
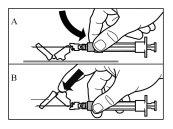
Siga cuidadosamente as instruções que se indicam a seguir para assegurar a reconstituição de Sandostatin LAR antes da injeção intramuscular profunda
Há três passos críticos no processo de reconstituição de Sandostatin LAR. Se não forem realizados corretamente, pode supor que o medicamento não é administrado de forma adequada.
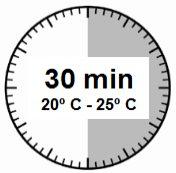
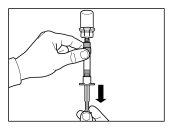
- O kit de injeção deve atingir a temperatura ambiente. Retirar o kit de injeção da geladeira e deixar que o kit atinja a temperatura ambiente durante um mínimo de 30 minutos antes da reconstituição, mas sem que se superem as 24 h.
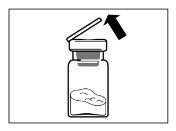
- Depois de adicionar a solução do diluente, deixar repousar o frasco durante 5 minutos para assegurar que o pó fica completamente saturado.
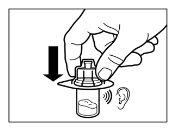
- Depois da saturação, agitar o frasco moderadamenteem direção horizontal durante um mínimo de 30 segundos até que se forme uma suspensão uniforme. A suspensão de Sandostatin LAR só deve ser preparada imediatamenteantes da administração.
Sandostatin LAR deve ser administrado apenas por um profissional de saúde especializado.
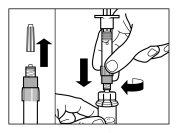
| PASSO 1
ATENÇÃO: é essencial iniciar o processo de reconstituição apenas após o kit de injeção ter atingido a temperatura ambiente. Deixar que o kit atinja a temperatura ambiente durante um mínimo de 30 minutos antes da reconstituição, mas não exceder as 24 h. Nota: O Kit de injeção pode ser refrigerado novamente, se necessário. |
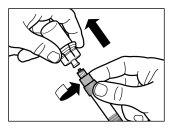
| PASSO 2
|
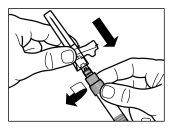
| PASSO 3
|
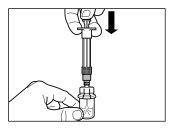
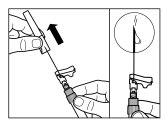
| |
| PASSO 4 ATENÇÃO: é essencial deixar repousar o frasco durante 5 minutospara assegurar que o diluente saturou completamente o pó. Nota: é normal que o êmbolo se desloque para cima, pois pode haver uma sobrepresão no frasco.
|
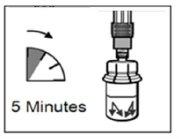
| PASSO 5
ATENÇÃO:Manter o êmbolo pressionado e agitar o frasco moderadamenteem sentido horizontal duranteum mínimo de 30 segundospara que o pó esteja completamente suspenso no diluente (suspensão uniforme de consistência leitosa). Repetir a agitação moderada durante outros 30 segundos mais se o pó não estiver completamente suspenso. |
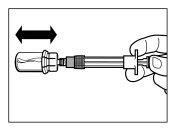
| PASSO 6
|
| |
| PASSO 7
|
| PASSO 8
|
| PASSO 9
|
- País de registo
- Substância ativa
- Requer receita médicaSim
- Fabricante
- Esta informação é apenas para referência e não constitui aconselhamento médico. Consulte sempre um médico antes de tomar qualquer medicamento. A Oladoctor não se responsabiliza por decisões médicas baseadas neste conteúdo.
- Alternativas a SANDOSTATIN LAR 30 mg PÓ E SOLVENTE PARA SUSPENSÃO INJETÁVELForma farmacêutica: INJETÁVEL, Octreotida 100 microgramas/mlSubstância ativa: octreotideFabricante: Gp Pharm S.A.Requer receita médicaForma farmacêutica: INJETÁVEL, Octreotida 500 microgramas/mlSubstância ativa: octreotideFabricante: Gp Pharm S.A.Requer receita médicaForma farmacêutica: INJETÁVEL, 200 microgramas/mlSubstância ativa: octreotideFabricante: Gp Pharm S.A.Requer receita médica
Alternativas a SANDOSTATIN LAR 30 mg PÓ E SOLVENTE PARA SUSPENSÃO INJETÁVEL noutros países
As melhores alternativas com o mesmo princípio ativo e efeito terapêutico.
Alternativa a SANDOSTATIN LAR 30 mg PÓ E SOLVENTE PARA SUSPENSÃO INJETÁVEL em Polonia
Alternativa a SANDOSTATIN LAR 30 mg PÓ E SOLVENTE PARA SUSPENSÃO INJETÁVEL em Ucrania
Médicos online para SANDOSTATIN LAR 30 mg PÓ E SOLVENTE PARA SUSPENSÃO INJETÁVEL
Avaliação de posologia, efeitos secundários, interações, contraindicações e renovação da receita de SANDOSTATIN LAR 30 mg PÓ E SOLVENTE PARA SUSPENSÃO INJETÁVEL – sujeita a avaliação médica e regras locais.