RESPREEZA 5 000 mg PÓ E SOLVENTE PARA SOLUÇÃO PARA PERFUSÃO
Pergunte a um médico sobre a prescrição de RESPREEZA 5 000 mg PÓ E SOLVENTE PARA SOLUÇÃO PARA PERFUSÃO

Como usar RESPREEZA 5 000 mg PÓ E SOLVENTE PARA SOLUÇÃO PARA PERFUSÃO
Introdução
Prospecto: Informação para o utilizador
Respreeza 1.000 mg pó e dissolvente para solução para perfusão
Respreeza 4.000 mg pó e dissolvente para solução para perfusão
Respreeza 5.000 mg pó e dissolvente para solução para perfusão
Inibidor de proteinase alfa1 humano
Leia todo o prospecto atentamente antes de começar a usar este medicamento, porque contém informações importantes para si.
- Conserva este prospecto, porque pode ter que voltar a lê-lo.
- Se tiver alguma dúvida, consulte o seu médico ou profissional de saúde.
- Este medicamento foi-lhe prescrito apenas a si, e não deve dá-lo a outras pessoas, mesmo que tenham os mesmos sintomas que si, porque pode prejudicá-las.
- Se experimentar efeitos adversos, consulte o seu médico ou profissional de saúde, mesmo que se trate de efeitos adversos que não aparecem neste prospecto. Ver a secção 4.
Conteúdo do prospecto:
- O que é Respreeza e para que é utilizado
- O que precisa saber antes de começar a usar Respreeza
- Como usar Respreeza
- Posíveis efeitos adversos
- Conservação de Respreeza
- Conteúdo do envase e informações adicionais
1. O que é Respreeza e para que é utilizado
O que é Respreeza
Este medicamento contém o princípio ativo inibidor de proteinase alfa1 humano que é um componente normal do sangue e encontra-se nos pulmões, onde a sua principal função é proteger o tecido pulmonar limitando a ação de uma certa enzima, chamada elastase neutrófila. Esta pode causar dano se a sua ação não for controlada (por exemplo, em caso de si padecer de um défice do inibidor de proteinase alfa1).
Para que é utilizado Respreeza
Este medicamento é utilizado em adultos com um défice grave conhecido do inibidor de proteinase alfa1 (uma afecção hereditária a que também se chama défice de antitripsina alfa1) que desenvolveram uma afecção pulmonar chamada enfisema.
Desenvolve-se enfisema quando a falta do inibidor de proteinase alfa1 afeta o controlo adequado da elastase neutrófila, o que danifica os pequenos sacos de ar nos pulmões através dos quais o oxigénio passa para o organismo. Devido a este dano, os pulmões não funcionam corretamente.
O uso regular deste medicamento aumenta as concentrações sanguíneas e pulmonares do inibidor de proteinase alfa1, diminuindo assim a progressão do enfisema.
2. O que precisa saber antes de começar a usar Respreeza
NÃO use Respreeza
- se é alérgico ao inibidor de proteinase alfa1 humano ou a qualquer um dos outros componentes deste medicamento (incluídos na secção 6).
- se foi determinado que si padece de alguma deficiência de certas proteínas sanguíneas chamadas imunoglobulinas do tipo A (IgA) e desenvolveu anticorpos contra elas.
Advertências e precauções
- Consulte o seu médico ou profissional de saúde antes de usar Respreeza.
Informação sobre reações alérgicas: quando é necessário parar ou diminuir a velocidade da perfusão?
É possível que si seja alérgico ao inibidor de proteinase alfa1 humano, mesmo que tenha recebido previamente inibidores de proteinase alfa1 humanos e os tenha tolerado bem. Em alguns casos, podem ocorrer reações alérgicas graves. O seu médico informá-lo-á sobre os sinais das reações alérgicas (por exemplo, arrepios, rubor, batimento cardíaco mais rápido, queda da pressão arterial, tontura, erupção, urticária, picazón, dificuldade para respirar ou engolir, bem como inchaço das mãos, face ou boca) (ver também a secção 4).
- Se si nota tais reações durante a perfusão do seu medicamento, avise o seu médico ou profissional de saúde imediatamente. Em função do tipo e da intensidade da reação, pode ser que o seu médico decida diminuir a velocidade ou parar completamente a perfusão e iniciar o tratamento apropriado.
- Em caso de autoadministração/tratamento domiciliário, pare a perfusão imediatamentee entre em contacto com o seu médico ou profissional de saúde.
Informação sobre segurança em relação às infecções
Respreeza é elaborado a partir de plasma de sangue humano (esta é a parte líquida do sangue da qual se eliminaram as células sanguíneas).
Devido a que através do sangue se podem transmitir infecções, quando se fabricam medicamentos a partir do sangue ou do plasma humano, são postas em prática certas medidas para evitar que estes estejam presentes no medicamento e sejam transmitidos aos pacientes. Entre tais medidas, incluem-se as seguintes:
- a seleção cuidadosa dos doadores de sangue e plasma com o fim de garantir a exclusão das pessoas que possam ser portadoras de infecções,
- a realização de testes das amostras de sangue e plasma doados para tratar de evitar o uso de material com sinais de vírus ou infecções,
- a inclusão de medidas no processamento do sangue ou do plasma que permitam inativar ou eliminar os vírus.
As medidas adotadas consideram-se eficazes para os vírus como o vírus da imunodeficiência humana (VIH), o vírus da hepatite A, o vírus da hepatite B, o vírus da hepatite C e o parvovirus B19.
No entanto, apesar destas medidas, ao administrar medicamentos preparados a partir de sangue ou plasma humano, não se pode excluir completamente a possibilidade de transmitir uma infecção. Isto também se aplica a qualquer vírus desconhecido ou emergente ou outros tipos de infecções.
O seu médico pode recomendar-lhe que considere a vacinação contra a hepatite A e B se receber um tratamento regular/repetido com inibidores de proteinase derivados de plasma humano.
- Recomenda-se encarecidamente que cada vez que receber uma dose de Respreeza se registe o nome e o número do lote do produto para manter um registo dos lotes utilizados.
Tabagismo
Devido a que o fumo do tabaco é um importante fator de risco para o desenvolvimento e a progressão do enfisema, recomenda-se encarecidamente que deixe de fumar e evite a exposição passiva ao fumo do tabaco.
Crianças e adolescentes
Este medicamento não é para uso em crianças ou adolescentes menores de 18 anos de idade.
Uso de Respreeza com outros medicamentos
- Informa o seu médico ou profissional de saúde se está a tomar, tomou recentemente ou pode ter que tomar qualquer outro medicamento.
Gravidez, lactação e fertilidade
- Se está grávida ou em período de lactação, acha que pode estar ou tem intenção de ficar grávida, consulte o seu médico ou profissional de saúde antes de utilizar este medicamento.
Como o inibidor de proteinase alfa1 é um componente normal do sangue humano, não se espera que a dose recomendada deste medicamento cause qualquer dano ao feto em desenvolvimento. No entanto, devido a que não se dispõe de informações sobre a segurança do uso de Respreeza durante a gravidez, se si está grávida, apenas se lhe deve administrar este medicamento com precaução.
Não se sabe se Respreeza passa para o leite materno. Se está a amamentar o seu filho, o seu médico explicar-lhe-á os riscos e benefícios de usar este medicamento.
Não existem dados sobre os efeitos na fertilidade, embora, como o inibidor de proteinase alfa1 é um componente normal do sangue humano, não se espera que cause efeitos adversos na fertilidade se si usar Respreeza na dose recomendada.
Condução e uso de máquinas
Pode ocorrer tontura após a administração deste medicamento. Se si sentir tontura, não deve conduzir nem usar máquinas até que a tontura tenha passado (ver secção 4).
Respreeza contém sódio
Respreeza 1.000 mg pó e dissolvente para solução para perfusão:
Este medicamento contém aproximadamente 37 mg de sódio (componente principal do sal de mesa/para cozinhar) em cada frasco de Respreeza 1.000 mg. Isto equivale a 1,9% da ingestão diária máxima de sódio recomendada para um adulto.
Respreeza 4.000 mg pó e dissolvente para solução para perfusão:
Este medicamento contém aproximadamente 149 mg de sódio (componente principal do sal de mesa/para cozinhar) em cada frasco de Respreeza 4.000 mg. Isto equivale a 7,4% da ingestão diária máxima de sódio recomendada para um adulto.
Respreeza 5.000 mg pó e dissolvente para solução para perfusão:
Este medicamento contém aproximadamente 186 mg de sódio (componente principal do sal de mesa/para cozinhar) em cada frasco de Respreeza 5.000 mg. Isto equivale a 9,3% da ingestão diária máxima de sódio recomendada para um adulto.
O seu médico ou profissional de saúde terá isto em conta se si seguir uma dieta com controlo de sódio.
3. Como usar Respreeza
Após a reconstituição, Respreeza é administrado por perfusão em uma veia. Um profissional de saúde com experiência no tratamento do défice de inibidor de proteinase alfa1 supervisionará as primeiras perfusões.
Tratamento domiciliário/Autoadministração
Após as primeiras perfusões, si ou uma pessoa encarregada dos seus cuidados também pode administrar Respreeza, mas apenas após receber uma formação adequada. Se o seu médico decidir que si é apto para tal tratamento domiciliário/autoadministração, ensinar-lhe-á sobre:
- como preparar e administrar este medicamento (ver as instruções ilustradas no final deste prospecto em “Informação para os profissionais de saúde e para os pacientes aptos para o tratamento domiciliário/autoadministração”)
- como manter o produto estéril (técnicas assépticas de perfusão)
- como manter um registo diário do tratamento
- como identificar os efeitos adversos, incluindo os sinais de reações alérgicas, e as medidas que devem ser tomadas em caso de que se manifestem tais efeitos (ver também a secção 2 e a secção 4)
O seu médico ou profissional de saúde reverá regularmente a sua técnica de perfusão ou da pessoa encarregada dos seus cuidados para assegurar que se segue a agir adequadamente.
Dose
A quantidade de Respreeza que lhe é administrada baseia-se no seu peso corporal. A dose recomendada é de 60 mg por kg de peso corporal e deve ser administrada uma vez por semana. A solução de perfusão normalmente é administrada durante cerca de 15 minutos (aproximadamente 0,08 ml de solução por kg de peso corporal cada minuto). Em função do seu peso e tolerabilidade à perfusão, o seu médico determinará a velocidade de perfusão apropriada para si.
Se usar mais Respreeza do que deve
Desconhecem-se as consequências de uma sobredose.
- Avise o seu médico ou profissional de saúde se pensar que usou mais Respreeza do que deve para que se tomem as medidas apropriadas.
Se esquecer de usar Respreeza
- Aplique imediatamente a próxima dose e continue a intervalos regulares seguindo as indicações do seu médico ou profissional de saúde.
- Não tome uma dose dupla para compensar a dose esquecida.
Se interromper o tratamento com Respreeza
- Não deixe de usar este medicamento sem consultar antes o seu médico ou profissional de saúde. Se se suspender o tratamento com Respreeza, a sua condição pode piorar.
4. Posíveis efeitos adversos
Como todos os medicamentos, este medicamento pode produzir efeitos adversos, embora nem todas as pessoas os sofram. Estas reações adversas podem ocorrer embora si tenha recebido previamente inibidores de proteinase alfa1 humana e os tenha tolerado bem.
Alguns efeitos adversos podem ser graves:
Foram observadas reações alérgicas pouco frequentes (podem afetar até 1 de cada 100 pessoas).
Em alguns casos muito raros (podem afetar até 1 de cada 10.000 pessoas) podem tornar-se reações alérgicas graves, mesmo que si não tenha mostrado sinais de alergia com perfusões previas.
- Avise o seu médico ou profissional de saúde imediatamentese si perceber qualquer sinal de reações alérgicas (por exemplo, arrepios, rubor, batimento cardíaco mais rápido, queda da pressão arterial, tontura, erupção, urticária, picazón, dificuldade para respirar ou engolir, bem como inchaço das mãos, face ou boca) durante a administração de Respreeza.
Em função do tipo e da intensidade da reação, pode ser que o seu médico ou profissional de saúde decida diminuir a velocidade ou parar completamente a administração e instituir o tratamento apropriado para a reação.
Em caso de autoadministração/tratamento domiciliário, pare a perfusão imediatamentee entre em contacto com o seu médico ou profissional de saúde.
Outros efeitos adversospodem incluir:
Frequentes(podem afetar até 1 de cada 10 pessoas)
Tonturas, dor de cabeça, dificuldade para respirar (dispnéia), náuseas.
Pouco frequentes(podem afetar até 1 de cada 100 pessoas)
Sensação alterada do tacto como ardor, picazón ou sensação de entorpecimento em suas mãos, braços, pernas ou pés (parestesia), rubor, urticária, erupção escamosa e erupção por todo o corpo, fraqueza física (astenia), reações no local da perfusão (como ardor, picadas, dor, inchaço ou rubor no local da perfusão (hematoma)).
Muito raros(podem afetar até 1 de cada 10.000 pessoas)
Sensação diminuída do tacto como ardor, picazón ou sensação de entorpecimento em suas mãos, braços, pernas ou pés (hipoestesia), sudorese excessiva (hiperhidrose), picazón, dor no peito, arrepios, febre (pirexia).
Frequência não conhecida(não pode ser estimada a partir dos dados disponíveis)
Dor em gânglios linfáticos (massas de tecido de forma ovalada que se localizam por todo o corpo e que podem ser palpadas, por exemplo, nas axilas, virilha ou pescoço), inchaço de face, olhos e lábios.
Comunicação de efeitos adversos
- Se experimentar qualquer tipo de efeito adverso, consulte o seu médico ou profissional de saúde, mesmo que se trate de efeitos adversos que não aparecem neste prospecto. Também pode comunicá-los directamente através do sistema nacional de notificação incluído no Apêndice V. Mediante a comunicação de efeitos adversos, si pode contribuir para fornecer mais informações sobre a segurança deste medicamento.
5. Conservação de Respreeza
Mantenha este medicamento fora da vista e do alcance das crianças.
Não utilize este medicamento após a data de validade que aparece na caixa e nas etiquetas dos frascos após EXP. A data de validade é o último dia do mês que se indica.
Não conserve a uma temperatura superior a 25 °C. Não congele.
Após a reconstituição, a solução deve ser utilizada imediatamente. Se isto não for possível, as soluções podem ser conservadas até 3 horas a temperatura ambiente (até 25 °C). Não congele a solução reconstituída.
6. Conteúdo do envase e informação adicional
Composição de Respreeza
O princípio ativoé o inibidor de proteinase alfa1 humano. Um frasco contém aproximadamente 1.000 mg, 4.000 mg ou 5.000 mg do inibidor de proteinase alfa1 humano.
Os demais componentessão cloreto de sódio, dihidrogenofosfato de sódio monohidratado e manitol (ver seção 2).
Veículo: água para preparações injetáveis.
Aspecto do produto e conteúdo do envase
Este medicamento é um pó de cor branca a esbranquiçada.
Depois de que seja reconstituído com água para preparações injetáveis, a solução deve ser transparente, incolor a ligeiramente amarelada e estar isenta de partículas visíveis.
Apresentações:
Conteúdo de um envase:
Respreeza 1.000 mg pó e veículo para solução para perfusão
- 1 frasco com pó de uso único
- 1 frasco de veículo com 20 ml de água para preparações injetáveis
- 1 transferidor 20/20 (Mix2Vial) para a reconstituição
Respreeza 4.000 mg pó e veículo para solução para perfusão
- 1 frasco com pó de uso único
- 1 frasco de veículo com 76 ml de água para preparações injetáveis
- 1 transferidor 20/20 (Mix2Vial) para a reconstituição
Equipamento de administração (caixa interior):
- 1 equipamento de perfusão IV
- 1 catéter de perfusão tipo palomita
- 3 toalhetas impregnadas de álcool
Respreeza 5.000 mg pó e veículo para solução para perfusão
- 1 frasco com pó de uso único
- 1 frasco de veículo com 95 ml de água para preparações injetáveis
- 1 transferidor 20/20 (Mix2Vial) para a reconstituição
Equipamento de administração (caixa interior):
- 1 equipamento de perfusão IV
- 1 catéter de perfusão tipo palomita
- 3 toalhetas impregnadas de álcool
Pode ser que apenas alguns tamanhos de envase sejam comercializados.
Título da autorização de comercialização e responsável pela fabricação
CSL Behring GmbH
Emil-von-Behring-Strasse 76
D-35041 Marburg
Alemanha
Pode solicitar mais informações sobre este medicamento dirigindo-se ao representante local do título da autorização de comercialização:
Bélgica CSL Behring NV Tel: +32 15 28 89 20 | Lituânia CentralPharma Communications UAB Tel: +370 5 243 0444 |
| Luxemburgo CSL Behring NV Tel: +32 15 28 89 20 |
República Tcheca CSL Behring s.r.o. Tel: +420 702 137 233 | Hungria CSL Behring Kft. Tel: +36 1 213 4290 |
Dinamarca CSL Behring AB Tlf: +46 8 544 966 70 | Malta AM Mangion Ltd. Tel: +356 2397 6333 |
Alemanha CSL Behring GmbH Tel: +49 6190 75 84810 | Países Baixos CSL Behring BV Tel: +31 85 111 96 00 |
Estônia CentralPharma Communications OÜ Tel: +3726015540 | Noruega CSL Behring AB Tlf: +46 8 544 966 70 |
Grécia CSL Behring ΕΠΕ Τηλ: +30 210 7255 660 | Áustria CSL Behring GmbH Tel: +43 1 80101 1040 |
Espanha CSL Behring S.A. Tel: +34 933 67 1870 | Polônia CSL Behring Sp. z.o.o. Tel: +48 22 213 22 65 |
França CSL Behring SA Tél: +33 1 53 58 54 00 | Portugal CSL Behring Lda Tel: +351 21 782 62 30 |
Croácia Marti Farm d.o.o. Tel: +385 1 5588297 | Romênia Prisum Healthcare S.R.L. Tel: +40 21 322 01 71 |
Irlanda CSL Behring GmbH Tel: +49 6190 75 84700 | Eslovênia EMMES BIOPHARMA GLOBAL s.r.o.-podružnica v Sloveniji Tel: +386 41 42 0002 |
Islândia CSL Behring AB Sími: +46 8 544 966 70 | República Eslovaca CSL Behring Slovakia s.r.o. Tel: +421 911 653 862 |
Itália CSL Behring S.p.A. Tel: +39 02 34964 200 | Finlândia CSL Behring AB Puh/Tel: +46 8 544 966 70 |
Chipre CSL Behring ΕΠΕ Τηλ: +30 210 7255 660 | Suécia CSL Behring AB Tel: +46 8 544 966 70 |
Letônia CentralPharma Communications SIA Tel: +371 6 7450497 |
Data da última revisão deste prospecto:
A informação detalhada deste medicamento está disponível no site da Agência Europeia de Medicamentos: http://www.ema.europa.eu.
--------------------------------------------------------------------------------------------------------------------
A seguinte informação é destinada a profissionais de saúde e a pacientes aptos para o tratamento domiciliar/autoadministração
Instruções gerais
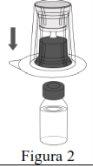
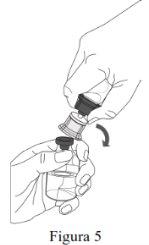
- A reconstituição deve ser realizada de acordo com as instruções fornecidas a seguir.
- O produto deve ser reconstituído, administrado e manipulado com precaução utilizando técnicas assépticas para manter a esterilidade do produto.
- Não use os acessórios auxiliares estéreis fornecidos para a reconstituição e administração se o seu envase estiver aberto ou se estiverem danificados.
- O pó deve ser reconstituído com o veículo (água para preparações injetáveis).
- A reconstituição total do pó deve ser realizada em 5 minutos (apresentação de 1.000 mg) ou em 10 minutos (apresentações de 4.000 mg e 5.000 mg).
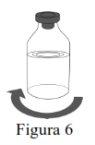
- Inspeccione a solução reconstituída em busca de partículas e decoloração antes da administração.
- A solução reconstituída deve ser transparente, incolor a ligeiramente amarelada e livre de partículas visíveis.
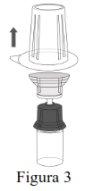
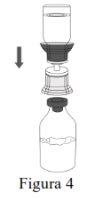
Siga os passos que se indicam a seguir para a preparação e reconstituição de Respreeza:
| |
| |
| |
Não retire o Mix2Vial do envase blíster. |
|
|
|
|
|
| |
| |
NOTA: Certifique-se de que toda a água tenha sido transferida para o frasco de Respreeza. |
|
Descarte o frasco de água para preparações injetáveis com todo o Mix2Vial. |
|
|
|
| |
Utilize um Mix2Vial separado e não utilizado, e um frasco de água para preparações injetáveis para cada frasco de Respreeza. | |
Utilize uma técnica asséptica para transferir a solução reconstituída para o contenedor de perfusão. |
Administração
A solução reconstituída deve ser administrada utilizando um equipamento de perfusão IV (fornecido com as apresentações de 4.000 mg e 5.000 mg).
|
|
|
|
|
|
|
|
|
Cada frasco de Respreeza é para uso único.
Todo o produto não utilizado ou material residual deve ser descartado seguindo as instruções do seu médico ou profissional de saúde.
- País de registo
- Substância ativa
- Requer receita médicaSim
- Fabricante
- Esta informação é apenas para referência e não constitui aconselhamento médico. Consulte sempre um médico antes de tomar qualquer medicamento. A Oladoctor não se responsabiliza por decisões médicas baseadas neste conteúdo.
- Alternativas a RESPREEZA 5 000 mg PÓ E SOLVENTE PARA SOLUÇÃO PARA PERFUSÃOForma farmacêutica: PERFURAÇÃO INJETÁVEL, 1000 mgSubstância ativa: alfa1 antitrypsinFabricante: Instituto Grifols S.A.Requer receita médicaForma farmacêutica: PERFURAÇÃO INJETÁVEL, 1000 mg alfa 1 antitripsinaSubstância ativa: alfa1 antitrypsinFabricante: Grifols Deutschland GmbhRequer receita médicaForma farmacêutica: PERFURAÇÃO INJETÁVEL, 4.000 mgSubstância ativa: alfa1 antitrypsinFabricante: Grifols Deutschland GmbhRequer receita médica
Alternativas a RESPREEZA 5 000 mg PÓ E SOLVENTE PARA SOLUÇÃO PARA PERFUSÃO noutros países
As melhores alternativas com o mesmo princípio ativo e efeito terapêutico.
Alternativa a RESPREEZA 5 000 mg PÓ E SOLVENTE PARA SOLUÇÃO PARA PERFUSÃO em Polónia
Alternativa a RESPREEZA 5 000 mg PÓ E SOLVENTE PARA SOLUÇÃO PARA PERFUSÃO em Ukraine
Médicos online para RESPREEZA 5 000 mg PÓ E SOLVENTE PARA SOLUÇÃO PARA PERFUSÃO
Avaliação de posologia, efeitos secundários, interações, contraindicações e renovação da receita de RESPREEZA 5 000 mg PÓ E SOLVENTE PARA SOLUÇÃO PARA PERFUSÃO – sujeita a avaliação médica e regras locais.