
POMBILITI 105 mg PÓ PARA CONCENTRADO PARA SOLUÇÃO PARA PERFUSÃO

Pergunte a um médico sobre a prescrição de POMBILITI 105 mg PÓ PARA CONCENTRADO PARA SOLUÇÃO PARA PERFUSÃO

Como usar POMBILITI 105 mg PÓ PARA CONCENTRADO PARA SOLUÇÃO PARA PERFUSÃO
Introdução
Prospecto: informação para o paciente
Pombiliti 105 mg pó para concentrado para solução para perfusão
cipaglucosidase alfa
Este medicamento está sujeito a acompanhamento adicional, o que agilizará a detecção de nova informação sobre a sua segurança. Pode contribuir comunicando os efeitos adversos que possa ter. A parte final da seção 4 inclui informação sobre como comunicar esses efeitos adversos.
Leia todo o prospecto atentamente antes de começar a receber este medicamento, porque contém informação importante para si.
- Conserva este prospecto, porque pode ter que voltar a lê-lo.
- Se tiver alguma dúvida, consulte o seu médico, farmacêutico ou enfermeiro.
- Se experimentar efeitos adversos, consulte o seu médico, farmacêutico ou enfermeiro, mesmo que se trate de efeitos adversos que não aparecem neste prospecto. Ver seção 4.
Conteúdo do prospecto
- O que é Pombiliti e para que é utilizado
- O que precisa saber antes de começar a receber Pombiliti
- Como é administrado Pombiliti
- Posíveis efeitos adversos
- Conservação de Pombiliti
- Conteúdo do envase e informação adicional
1. O que é Pombiliti e para que é utilizado
Pombiliti é um tipo de “terapia de substituição enzimática” (TSE) que está indicado para adultos com doença de Pompe de início tardio. Contém o princípio ativo chamado “cipaglucosidase alfa”.
Para que é utilizado
Pombiliti é utilizado sempre junto com outro medicamento chamado miglustato 65 mg cápsulas duras. É muito importante que você também leia o prospecto de miglustato 65 mg cápsulas duras.
Se tiver alguma dúvida sobre esses medicamentos, consulte o seu médico ou farmacêutico.
Como age Pombiliti
As pessoas com a doença de Pompe têm níveis baixos de uma enzima chamada α-glucosidase ácida (GAA). Essa enzima ajuda a regular os níveis de glicogênio (um tipo de hidrato de carbono) no organismo.
Na doença de Pompe, acumulam-se grandes quantidades de glicogênio nos músculos de todo o corpo. Isso impede o funcionamento correto dos músculos, por exemplo, os que ajudam a caminhar, os que facilitam a respiração nos pulmões e o músculo cardíaco.
Pombiliti entra nas células musculares que estão afetadas pela doença de Pompe. Uma vez no interior das células, o medicamento age como a GAA, favorecendo a decomposição do glicogênio e regulando os seus níveis.
2. O que precisa saber antes de começar a receber Pombiliti
Não deve receber Pombiliti
- Se já teve reações de hipersensibilidade potencialmente mortais ao seguinte:
- cipaglucosidase alfa
- miglustato
- algum dos outros componentes deste medicamento (incluídos na seção 6).
- Se uma perfusão anterior teve que ser interrompida e não pôde ser retomada devido a reações de hipersensibilidade potencialmente mortais.
Advertências e precauções
Consulte o seu médico, farmacêutico ou enfermeiro antes de começar a usar Pombiliti
Consulte o seu médico ou enfermeiro imediatamentese alguma dessas situações for aplicável ao seu caso, ou acredite que possam ser, ou se já teve alguma dessas reações com outra terapia de substituição enzimática (TSE):
- reações alérgicas, incluindo a anafilaxia (uma reação alérgica grave) - ver a seção 4 do apartado “Posíveis efeitos adversos”, mais abaixo, para conhecer os sintomas das reações potencialmente mortais;
- reação associada à perfusão enquanto está recebendo o medicamento ou nas horas posteriores, ver seção 4 do apartado “Posíveis efeitos adversos”, mais abaixo, para conhecer os sintomas das reações potencialmente mortais.
Informa ao seu médico se tem antecedentes de alguma doença cardíaca ou pulmonar. Essas doenças podem piorar durante ou imediatamente após a perfusão de Pombiliti. Informa ao seu médico ou enfermeiro imediatamente se sofre dificuldade para respirar, tosse, batimentos rápidos ou irregulares do coração ou qualquer outro efeito dessas doenças.
Informa também ao médico se apresenta inchaço nas pernas ou inchaço generalizado do corpo, erupção cutânea grave ou urina espumosa ao eliminar líquidos. O médico decidirá se a perfusão de Pombiliti deve ser interrompida e lhe dará o tratamento médico adequado. Assim, o médico decidirá se pode continuar a receber Pombiliti.
Medicamentos antes do tratamento
É possível que o seu médico lhe administre outros medicamentos antes do tratamento com Pombiliti, por exemplo:
- antihistamínicos e corticosteroides para prevenir ou mitigar as reações associadas à perfusão;
- antipiréticos para reduzir a febre.
Crianças e adolescentes
Este medicamento não deve ser administrado a pacientes menores de 18 anos, porque se desconhecem os efeitos de Pombiliti junto com miglustato neste grupo de idade.
Outros medicamentos e Pombiliti
Informa ao seu médico ou enfermeiro se está utilizando, utilizou recentemente ou possa ter que utilizar qualquer outro medicamento, incluindo os medicamentos adquiridos sem receita e os medicamentos à base de plantas.
Gravidez e lactação
Se está grávida ou em período de lactação, acredita que possa estar grávida ou tem intenção de ficar grávida, não deve receber este medicamento, mas sim consultar o seu médico ou farmacêutico imediatamente antes de utilizá-lo.
Não há dados sobre o uso de Pombiliti em combinação com miglustato durante a gravidez.
- Se está grávida, não deve receber Pombiliti e/ou tomar miglustato 65 mg cápsulas duras. Informa ao seu médico imediatamente se fica grávida, acredita que possa estar grávida ou tem intenção de ficar grávida. Pode haver riscos para o bebê em gestação.
- Pombiliti em combinação com miglustato não deve ser administrado a mulheres em período de lactação. Deve-se decidir se é necessário deixar a lactação ou deixar o tratamento.
Anticoncepção e fertilidade
As mulheres em idade fértil devem utilizar métodos anticonceptivos eficazes, durante e 4 semanas após o fim da administração de ambos os medicamentos.
Condução e uso de máquinas
É possível que tenha tonturas, sonolência ou pressão arterial baixa (hipotensão) após receber Pombiliti ou os medicamentos antes do tratamento. Nesse caso, não conduza nem utilize ferramentas ou máquinas.
Pombiliti contém sódio
Este medicamento contém 10,5 mg de sódio (componente principal da sal de mesa/para cozinhar) em cada frasco. Isso equivale a 0,52% da ingestão diária máxima de sódio recomendada para um adulto.
3. Como é administrado Pombiliti
Pombiliti é administrado por um médico ou enfermeiro. É administrado em forma de gotejamento no interior de uma veia. Esta forma de administração é denominada perfusão intravenosa.
Consulte o seu médico se deseja tratar-se em casa. Este decidirá, após a avaliação, se é seguro que receba a perfusão de Pombiliti em sua casa. Se experimentar algum efeito secundário durante uma perfusão de Pombiliti, é possível que o membro do pessoal encarregado de administrá-la detenha a perfusão e inicie o tratamento farmacológico que corresponda.
Pombiliti deve ser utilizado em combinação com miglustato. Só pode utilizar miglustato 65 mg cápsulas com cipaglucosidase alfa. NÃOutilize miglustato 100 mg cápsulas (medicamento diferente). Com respeito à dose recomendada, siga as instruções do seu médico e leia o prospecto de miglustato 65 mg cápsulas duras.
Qual a quantidade de Pombiliti administrada
A quantidade de medicamento que receberá é baseada no seu peso. A dose recomendada é de 20 mg por cada quilograma de peso corporal.
Quando é administrado Pombiliti e por quanto tempo
- Receberá tratamento com Pombiliti uma vez cada duas semanas. Miglustato 65 mg cápsulas é tomado no mesmo dia da administração de Pombiliti. Consulte o prospecto de miglustato 65 mg cápsulas duras para obter informação sobre como tomar miglustato.
- A perfusão de cipaglucosidase alfa deve começar 1 hora após ter tomado miglustato 65 mg cápsulas duras.
- Em caso de demora, o início da perfusão não deve exceder as 3 horas desde a tomada de miglustato.
- A perfusão de cipaglucosidase alfa dura cerca de 4 horas.
Figura 1. Desenvolvimento cronológico das doses
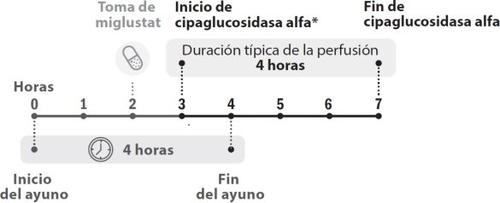
- A perfusão de cipaglucosidase alfa deve ser iniciada 1 hora após ter tomado as cápsulas de miglustato. Em caso de atraso na perfusão, o início desta não deve exceder as 3 horas desde a tomada de miglustato.
Mudança desde outra terapia de substituição enzimática (TSE)
Se atualmente está recebendo outra TSE:
- Seu médico lhe indicará quando deve deixar a outra TSE antes de iniciar Pombiliti.
- Informa ao seu médico de quando recebeu a última dose.
Se receber mais Pombiliti do que deve
Se tem dificuldade para respirar, sente inchaço ou inflamação, ou nota o coração acelerado, pode ser que tenha recebido demasiado Pombiliti; informa ao seu médico imediatamente. O excesso da velocidade de perfusão de Pombiliti pode provocar sintomas devido a um excesso de líquido no organismo, por exemplo, dificuldade para respirar, frequência cardíaca alta ou inchaço generalizado em todo o corpo.
Se esquecer a sua dose de Pombiliti
Se saltou uma perfusão, entre em contato com o seu médico ou enfermeiro o mais rápido possível, para marcar uma consulta e que possam administrar Pombiliti em combinação com miglustato 24 horas após a última tomada de miglustato.
Se interromper o tratamento com Pombiliti
Fale com o seu médico se deseja interromper o tratamento com Pombiliti. Os sintomas da sua doença podem piorar se interromper o tratamento.
4. Posíveis efeitos adversos
Como todos os medicamentos, este medicamento pode produzir efeitos adversos, embora nem todas as pessoas os sofram.
Pombiliti é utilizado junto com miglustato e qualquer um desses medicamentos pode produzir efeitos adversos. Os efeitos adversos foram observados principalmente nos pacientes durante a perfusão de Pombiliti (reações associadas à perfusão) ou pouco depois. Informa ao seu médico imediatamente se sofre uma reação associada à perfusão ou uma reação alérgica. Algumas dessas reações podem ser graves e potencialmente mortais. É possível que o seu médico lhe administre medicamentos antes da perfusão para prevenir essas reações.
Reações associadas à perfusão
A maioria das reações associadas à perfusão é leve ou moderada. Os sintomas de uma reação associada à perfusão são, entre outros, dificuldade para respirar, inchaço, febre, calafrios, tonturas, rubor e coceira na pele e erupção cutânea.
Reações alérgicas
As reações alérgicas podem dar origem a sintomas como erupção cutânea em qualquer parte do corpo, inchaço dos olhos, dificuldade para respirar prolongada, tosse, inchaço dos lábios, da língua ou da garganta, coceira na pele e urticária.
Muito frequentes(podem afetar mais de 1 de cada 10 pessoas)
- Dor de cabeça
Frequentes(podem afetar até 1 de cada 10 pessoas)
- Tosse
- Rubor súbito do rosto, do pescoço ou da parte superior do peito
- Dor no peito
- Erupção cutânea, coceira
- Aumento da pressão arterial
- Sudorese
- Inchaço abdominal
- Flatulência ou gases
- Diarréia, fezes soltas
- Vômitos
- Náuseas
- Febre ou calafrios
- Urticária
- Inchaço ou dor na zona de inserção da agulha
- Cãibras, dor ou fraqueza muscular
- Tremores em uma ou várias partes do corpo
- Aumento da sudorese
- Dor
- Alteração do sentido do gosto
- Sensação de cansaço constante ou de sono
- Dificuldade para respirar
Pouco frequentes(podem afetar até 1 de cada 100 pessoas)
- Respiração difícil e que provoca tosse, silbidos (sibilâncias) ao respirar e sensação de falta de ar (asma)
- Reação alérgica
- Inchaço das mãos, dos pés, dos tornozelos, das pernas
- Inchaço do rosto
- Dispepsia
- Dor de estômago
- Sensação de cansaço constante
- Dor ou irritação de garganta
- Contrações dolorosas e anormais da garganta
- Irritação da boca
- Dor na boca ou desconforto na parte de trás da boca
- Dor nas bochechas, nas gengivas, nos lábios, no queixo
- Perda de força e energia, sensação de fraqueza
- Mal-estar, sensação geral de letargia
- Sensação de ardor
- Raspados ou lesões na pele
- Alterações da temperatura corporal
- Diminuição de um tipo de glóbulo branco (detectada nos exames)
- Sonolência
- Tonturas
- Dor nas articulações
- Dor na zona entre a anca e as costelas
- Fadiga muscular
- Maior rigidez dos músculos
- Incapacidade para manter o equilíbrio
- Pressão arterial baixa
- Sensação de estar a ponto de desmaiar
- Dor de cabeça, dor pontiaguda, aura, dor nos olhos, sensibilidade à luz (enxaqueca)
- Manchas na pele
Comunicação de efeitos adversos
Se experimentar algum tipo de efeito adverso, consulte o seu médico, farmacêutico ou enfermeiro, mesmo que se trate de possíveis efeitos adversos que não aparecem neste prospecto. Também pode comunicá-los diretamente através do sistema nacional de notificação incluído no Apêndice V. Mediante a comunicação de efeitos adversos, você pode contribuir para fornecer mais informação sobre a segurança deste medicamento.
5. Conservação de Pombiliti
Seu médico, farmacêutico ou enfermeiro é o responsável por conservar este medicamento e por descartar corretamente os frascos abertos. Esta informação é destinada apenas a profissionais de saúde.
Mantenha este medicamento fora da vista e do alcance das crianças.
Não utilize este medicamento após a data de validade que aparece no frasco e na caixa após “CAD”. A data de validade é o último dia do mês que se indica.
Frascos não abertos: Conservar em geladeira (entre 2 ºC e 8 ºC). Conservar o frasco no embalagem exterior para protegê-lo da luz.
Após a diluição, recomenda-se uso imediato. No entanto, a conservação da bolsa de perfusão intravenosa com Pombiliti foi demonstrada durante 6 horas a uma temperatura entre 20 ºC e 25ºC e durante 24 horas a uma temperatura entre 2 ºC e 8 ºC.
Os medicamentos não devem ser jogados nos esgotos nem na lixeira. Pergunte ao seu farmacêutico como se livrar dos envases e dos medicamentos que já não precisa. Dessa forma, ajudará a proteger o meio ambiente.
6. Conteúdo do envase e informação adicional
Composição de Pombiliti
O princípio ativo é cipaglucosidase alfa. Um frasco contém 105 mg de cipaglucosidase alfa. Após a reconstituição, a solução contida no frasco contém 15 mg de cipaglucosidase alfa por mililitro. Recomenda-se uma concentração final de cipaglucosidase alfa diluída na bolsa de perfusão intravenosa de 0,5 mg/ml a 4 mg/ml.
Os outros componentes são:
- Citrato de sódio diidratado (E331)
- Ácido cítrico monoidratado (E330)
- Manitol (E421)
- Polissorbato 80 (E433)
Aspecto do produto e conteúdo do envase
Pombiliti é um pó branco a ligeiramente amarelo. Após a reconstituição, é uma solução de transparente a opalescente, entre incolor e ligeiramente amarela, sem partículas estranhas e praticamente livre de partículas de cor branca a translúcidas. A solução reconstituída deve ser diluída posteriormente em uma bolsa de perfusão intravenosa.
Pombiliti é um pó para concentrado para solução para perfusão em um frasco.
Envases de 1, 10 ou 25 frascos
Pode ser que apenas alguns tamanhos de envases sejam comercializados.
Título da autorização de comercialização
Amicus Therapeutics Europe Limited
Block 1, Blanchardstown Corporate Park
Ballycoolin Road
Blanchardstown, Dublin
D15 AKK1
Irlanda
Tel.: +353 (0) 1 588 0836
Fax: +353 (0) 1 588 6851
Correio eletrônico: [email protected]
Responsável pela fabricação
Manufacturing Packaging Farmaca (MPF) B.V.
Neptunus 12, Heerenveen, 8448CN, Países Baixos
Podem solicitar mais informações sobre este medicamento dirigindo-se ao representante local do titular da autorização de comercialização:
Bélgica Amicus Therapeutics Europe Limited Tel: (+32) 0800 89172 e-mail: [email protected] | Lituânia Amicus Therapeutics Europe Limited Tel: (+370) 8800 33167 E-mail: [email protected] |
| Luxemburgo Amicus Therapeutics Europe Limited Tel: (+352) 800 27003 e-mail: [email protected] |
República Tcheca Amicus Therapeutics Europe Limited Tel.: (+420) 800 142 207 e-mail: [email protected] | Hungria Amicus Therapeutics Europe Limited Tel.: (+36) 06 800 21202 e-mail: [email protected] |
Dinamarca Amicus Therapeutics Europe Limited Tel.: (+45) 80 253 262 e-mail: [email protected] | Malta Amicus Therapeutics Europe Limited Tel: (+356) 800 62674 e-mail: [email protected] |
Alemanha Amicus Therapeutics GmbH Tel: (+49) 0800 000 2038 E-Mail: [email protected] | Países Baixos Amicus Therapeutics BV Tel: (+31) 20 235 8510/(+31) 0800 022 8399 e-mail: [email protected] |
Estônia Amicus Therapeutics Europe Limited Tel: (+372) 800 0111 911 e-mail: [email protected] | Noruega Amicus Therapeutics Europe Limited Tel: (+47) 800 13837 e-mail: [email protected] |
Grécia Amicus Therapeutics Europe Limited Tel: (+30) 00800 126 169 e-mail: [email protected] | Áustria Amicus Therapeutics Europe Limited Tel: (+43) 0800 909 639 e-mail: [email protected] |
Espanha Amicus Therapeutics S.L.U. Tel: (+34) 900 941 616 e-mail: [email protected] | Polônia Amicus Therapeutics Europe Limited Tel.: (+48) 0080 012 15475 e-mail: [email protected] |
França Amicus Therapeutics SAS Tel: (+33) 0 800 906 788 e-mail: [email protected] | Portugal Amicus Therapeutics Europe Limited Tel: (+351) 800 812 531 e-mail: [email protected] |
Croácia Amicus Therapeutics Europe Limited Tel: (+358) 0800 222 452 e-mail: [email protected] | Irlanda Amicus Therapeutics Europe Limited Tel: (+353) 1800 936 230 e-mail: [email protected] |
Romênia Amicus Therapeutics Europe Limited Tel.: (+40) 0808 034 288 e-mail: [email protected] | Eslovênia Amicus Therapeutics Europe Limited Tel.: (+386) 0800 81794 e-mail: [email protected] |
Islândia Amicus Therapeutics Europe Limited Tel: (+354) 800 7634 e-mail: [email protected] | República Eslovaca Amicus Therapeutics Europe Limited Tel: (+421) 0800 002 437 e-mail: [email protected] |
Itália Amicus Therapeutics S.r.l. Tel: (+39) 800 795 572 e-mail: [email protected] | Finlândia Amicus Therapeutics Europe Limited Tel: (+358) 0800 917 780 e-mail: [email protected] |
Chipre Amicus Therapeutics Europe Limited Tel: (+357) 800 97595 e-mail: [email protected] | Suécia Amicus Therapeutics Europe Limited Tel: (+46) 020 795 493 e-mail: [email protected] |
Letônia Amicus Therapeutics Europe Limited Tel: (+371) 800 05391 e-mail: [email protected] | Reino Unido (Irlanda do Norte) Amicus Therapeutics, UK Limited Tel: (+44) 08 0823 46864 e-mail: [email protected] |
Data da última revisão deste prospecto
Outras fontes de informação
A informação detalhada sobre este medicamento está disponível no site da Agência Europeia de Medicamentos: http://www.ema.europa.eu. Também existem links para outros sites sobre doenças raras e medicamentos órfãos.
Esta informação é destinada apenas a profissionais de saúde:
Instruções de uso: reconstituição, diluição e administração
Pombiliti deve ser reconstituído com água para preparações injetáveis e, em seguida, diluído em uma solução de cloreto de sódio 9 mg/ml (0,9%) para preparações injetáveis e administrado por perfusão intravenosa. A reconstituição e diluição devem ser realizadas de acordo com as normas de boa prática clínica, especialmente no que diz respeito ao respeito à assepsia.
Como este medicamento é uma proteína, é possível que se formem partículas na solução reconstituída e na bolsa de perfusão diluída final. Portanto, deve ser utilizado um filtro em linha de 0,2 micrómetros de baixa ligação a proteínas para a administração. Foi demonstrado que o uso de um filtro em linha de 0,2 micrómetros elimina as partículas visíveis e não provoca uma perda aparente de proteínas nem de atividade.
Determine o número de frascos que devem ser reconstituídos com base na posologia do paciente (mg/kg) e retire os frascos necessários da geladeira para que atinjam a temperatura ambiente (cerca de 30 minutos). Cada frasco de Pombiliti é de uso único.
Use uma técnica asséptica.
Reconstituição
Reconstitua os 105 mg por frasco de Pombiliti em 7,2 ml de água para preparações injetáveis utilizando uma seringa com um diâmetro de agulha não superior a 18 G. Adicione a água para preparações injetáveis gota a gota pelo lado do frasco e não diretamente sobre o pó liofilizado. Incline e gire cada frasco com cuidado. Não inverta, remova nem agite o frasco. O volume de extração é uma solução de transparente a opalescente, entre incolor e ligeiramente amarela, sem partículas estranhas e praticamente livre de partículas de cor branca a translúcidas. Realize uma inspeção imediata dos frascos reconstituídos para verificar se não há partículas nem alteração da cor. Se na inspeção imediata forem observadas partículas estranhas distintas das descritas anteriormente ou a solução reconstituída apresentar uma alteração da cor, não a utilize. O pH da solução reconstituída é de aproximadamente 6,0.
Após a reconstituição, recomenda-se diluir os frascos imediatamente (ver abaixo).
Diluição
Após a reconstituição descrita anteriormente, a solução reconstituída no frasco contém 15 mg de cipaglucosidase alfa por mililitro. O volume reconstituído permite a extração exata de 7,0 ml (equivalente a 105 mg) de cada frasco. A solução deve ser diluída posteriormente da seguinte forma: com uma seringa de diâmetro de agulha não superior a 18 G, extraia lentamente a solução reconstituída de cada frasco, incluindo o volume inferior a 7,0 ml do frasco parcial, até obter a dose do paciente. A concentração final recomendada de cipaglucosidase alfa nas bolsas de perfusão está compreendida entre 0,5 mg/ml e 4 mg/ml. Extraia o ar do interior da bolsa de perfusão.
Da mesma forma, extraia um volume equivalente de solução injetável de cloreto de sódio 9 mg/ml (0,9%), que será substituído pelo volume de Pombiliti reconstituído. Injete lentamente a solução reconstituída de Pombiliti diretamente na solução de cloreto de sódio 9 mg/ml (0,9%) para preparações injetáveis. Inverta ou massageie com cuidado a bolsa de perfusão para misturar a solução diluída. Não sacuda nem agite excessivamente a bolsa de perfusão.
A solução final para perfusão deve ser administrada o mais rápido possível após a preparação.
A eliminação do medicamento não utilizado e de todos os materiais que estiveram em contato com ele será realizada de acordo com a regulamentação local.
Administração
A perfusão de Pombiliti deve começar 1 hora após a ingestão das cápsulas de miglustate. Em caso de atraso da perfusão, seu início não deve exceder 3 horas desde a ingestão de miglustate. A posologia recomendada de Pombiliti é de 20 mg/kg de peso corporal administrados a cada duas semanas por perfusão intravenosa.
As perfusões devem ser administradas de forma gradual. Recomenda-se que a velocidade inicial da perfusão seja de 1 mg/kg/h e vá aumentando gradualmente em 2 mg/kg/h a cada 30 minutos, se não aparecerem sinais de RAP (reações associadas à perfusão), até que se atinja uma velocidade máxima de 7 mg/kg/h.
- País de registo
- Substância ativa
- Requer receita médicaSim
- Fabricante
- Esta informação é apenas para referência e não constitui aconselhamento médico. Consulte sempre um médico antes de tomar qualquer medicamento. A Oladoctor não se responsabiliza por decisões médicas baseadas neste conteúdo.
- Alternativas a POMBILITI 105 mg PÓ PARA CONCENTRADO PARA SOLUÇÃO PARA PERFUSÃOForma farmacêutica: SOLUÇÃO INJETÁVEL PARA PERFUSÃO, 100 USubstância ativa: laronidaseFabricante: Sanofi B.V.Requer receita médicaForma farmacêutica: PERFURAÇÃO INJETÁVEL, 30 mg/mlSubstância ativa: cerliponase alfaFabricante: Biomarin International LimitedRequer receita médicaForma farmacêutica: PERFURAÇÃO INJETÁVEL, DesconhecidaSubstância ativa: imigluceraseFabricante: Sanofi B.V.Requer receita médica
Médicos online para POMBILITI 105 mg PÓ PARA CONCENTRADO PARA SOLUÇÃO PARA PERFUSÃO
Avaliação de posologia, efeitos secundários, interações, contraindicações e renovação da receita de POMBILITI 105 mg PÓ PARA CONCENTRADO PARA SOLUÇÃO PARA PERFUSÃO – sujeita a avaliação médica e regras locais.















