
MIRTAZAPINA TARBIS 30 mg COMPRIMIDOS ORODISPERSÍVEIS

Pergunte a um médico sobre a prescrição de MIRTAZAPINA TARBIS 30 mg COMPRIMIDOS ORODISPERSÍVEIS

Como usar MIRTAZAPINA TARBIS 30 mg COMPRIMIDOS ORODISPERSÍVEIS
Introdução
Prospecto: informação para outilizador
Mirtazapina TARBIS 30mgcomprimidos bucodispersáveis EFG
Leia todo o prospecto detenidamente antes de começar a tomar este medicamento, porque contém informações importantes para si.
- Conserva este prospecto, porque pode ter que voltar a lê-lo.
- Se tiver alguma dúvida, consulte o seu médico ou farmacêutico.
- Este medicamento foi-lhe prescrito apenas para si e não deve dá-lo a outras pessoas, mesmo que tenham os mesmos sintomas que si, porque pode prejudicá-las.
- Se experimentar efeitos adversos, consulte o seu médico ou farmacêutico, mesmo que se trate de efeitos adversos que não aparecem neste prospecto.
Conteúdo do prospecto
- O que é Mirtazapina Tarbis e para que é utilizado
- O que necessita saber antes de começar a tomar Mirtazapina Tarbis
- Como tomar Mirtazapina Tarbis
- Possíveis efeitos adversos
- Conservação de Mirtazapina Tarbis
- Conteúdo do envase e informações adicionais
1. O que é Mirtazapina Tarbis e para que é utilizado
Mirtazapina Tarbis pertence ao grupo de medicamentos chamados antidepressivos.
Mirtazapina é utilizado para tratar a depressão.
2. O que necessita saber antes de começar a tomar Mirtazapina Tarbis
Não tome MirtazapinaTarbis
- se é alérgico (hipersensível) ao princípio ativo ou a qualquer um dos outros componentes deste medicamento (incluídos na seção 6). Nesse caso, consulte o seu médico o mais breve possível antes de tomar Mirtazapina Tarbis.
- se está tomando ou tomou recentemente (nas duas últimas semanas) medicamentos chamados inibidores da monoaminooxidase (IMAO).
NÃO TOMAR - OU CONSULTE O SEU MÉDICO ANTES DE COMEÇAR A TOMAR MIRTAZAPINA TARBIS:
Se sofreu alguma vez uma erupção cutânea intensa ou descamação da pele, ampolas ou úlceras na boca após tomar mirtazapina ou outros medicamentos.
Advertências e precauções
Consulte o seu médico ou farmacêutico antes de começar a tomar Mirtazapina Tarbis.
Uso em crianças e adolescentes menores de 18 anos
Mirtazapina Tarbis não deve ser utilizado normalmente no tratamento de crianças e adolescentes menores de 18 anos. Ao mesmo tempo, deve saber que em pacientes menores de 18 anos existe um maior risco de efeitos adversos como tentativas de suicídio, ideação suicida e hostilidade (predominantemente agressividade, comportamento de confronto e irritabilidade) quando tomam esta classe de medicamentos. Apesar disso, o médico pode prescrever Mirtazapina Tarbis a pacientes menores de 18 anos quando decidir que é o mais conveniente para o paciente. Se o médico prescreveu Mirtazapina Tarbis a um paciente menor de 18 anos e deseja discutir esta decisão, por favor, volte ao seu médico. Deve informar o seu médico se aparecem ou pioram algum dos sintomas que se detalham anteriormente em pacientes menores de 18 anos que estão tomando Mirtazapina Tarbis. Além disso, ainda não se conhecem os efeitos sobre a segurança a longo prazo relacionados com o crescimento, a maturidade e o desenvolvimento do conhecimento e do comportamento de mirtazapina neste grupo de idade.
Pensamentos suicidas e piora da sua depressão ou transtorno de ansiedade
Se se encontra deprimido e/ou sofre transtorno de ansiedade, pode em algumas ocasiões ter pensamentos de autolesão ou de suicídio. Estes podem ir aumentando ao tomar antidepressivos pela primeira vez, posto que estes medicamentos requerem um tempo para começar a fazer efeito, geralmente ao redor de duas semanas, embora em alguns casos pudesse ser maior o tempo.
Será mais propenso a ter este tipo de pensamentos:
- Se previamente teve pensamentos de autolesão ou de suicídio.
- Se é um adulto jovem. Informação de ensaios clínicos demonstrou um aumento do risco de comportamentos suicidas em adultos jovens (menores de 25 anos) com doenças psiquiátricas e que foram tratados com um antidepressivo.
?Se em qualquer momento tem pensamentos de autolesão ou de suicídio, consulte o seu médico ou dirija-se diretamente a um hospital.
Pode ser de ajuda para si dizer a um parente ou um amigo próximoque se encontra deprimido ou que tem um transtorno de ansiedade, e pedir-lhe que leia este prospecto. Pode perguntar-lhes se pensam que a sua depressão ou transtorno de ansiedade piorou, ou se estão preocupados com mudanças no seu comportamento.
Assim como, tenha especial cuidado com Mirtazapina Tarbis:
- se tem ou teve alguma vez um dos seguintes transtornos
?Informe o seu médico sobre estas situações antes de tomar Mirtazapina Tarbis, se não o fez ainda:
- convulsões(epilepsia). Se aparecem convulsões ou as suas convulsões são mais frequentes, deixe de tomar mirtazapina e contacte com o seu médico imediatamente;
- doenças do fígado, incluindo icterícia. Se aparece icterícia, deixe de tomar mirtazapina e contacte com o seu médico imediatamente;
- doenças dos rins;
- doença do coraçãoou pressão arterial baixa;
- esquizofrenia. Se os sintomas psicóticos, como os pensamentos paranoicos, são mais frequentes ou graves contacte com o seu médico imediatamente;
- depressão bipolar(se alternam períodos de animação/hiperatividade e períodos de depressão). Se começa a se sentir animado ou sobreexcitado, deixe de tomar mirtazapina e contacte com o seu médico imediatamente;
- diabetes(poderia necessitar ajustar a sua dose de insulina ou outros medicamentos antidiabéticos);
- doenças dos olhos, como aumento da pressão no olho (glaucoma);
- dificuldades para urinar, que poderia dever-se a um aumento do tamanho da próstata;
- se aparecem sinais de infecção como febre alta inexplicável, dor de garganta e úlceras na boca
?Deixe de tomar Mirtazapina Tarbis e contacte com o seu médico imediatamente para realizar uma análise de sangue. Em raros casos, estes sintomas podem ser sinais de alterações na produção de células sanguíneas na medula óssea. Embora raros, estes sintomas aparecem às 4-6 semanas de tratamento.
- se é uma pessoa idosa poderia ser mais sensível aos efeitos adversos dos medicamentos antidepressivos.
- foram notificados com o uso de mirtazapina reações cutâneas graves, como síndrome de Stevens-Johnson (SSJ), necrólise epidérmica tóxica (NET) e reações medicamentosas com eosinofilia e sintomas sistémicos (DRESS). Interrompa o uso e procure atendimento médico imediatamente se notar algum dos sintomas descritos na seção 4 em relação a estas reações cutâneas graves.
- Se sofreu alguma vez reações cutâneas graves, não deve reiniciar o tratamento com mirtazapina.
Uso de Mirtazapina Tarbis com outros medicamentos
Comunique ao seu médico ou farmacêutico se está tomando (ou vai tomar) algum dos medicamentos da seguinte lista.
Comunique também ao seu médico ou farmacêutico que está utilizando, utilizou recentemente ou poderia ter que utilizar qualquer outro medicamento.
Não tome Mirtazapina Tarbisjunto com:
- inibidores da monoamino oxidase(inibidores da MAO). Assim como, não tome mirtazapina durante as duas semanas após ter deixado de tomar os inibidores da MAO. Se deixar de tomar mirtazapina, também não tome inibidores da MAO durante as duas semanas seguintes.
Exemplos de inibidores da MAO são moclobemida, tranilcipromina (ambos são antidepressivos) e selegilina (para a doença de Parkinson).
Tenha cuidado setoma Mirtazapina Tarbis junto com:
- antidepressivos como os inibidores seletivos da recaptura de serotonina (ISRSs), venlafaxina e L-triptófano ou triptanos(utilizados para a enxaqueca), tramadol(para a dor), linezolida(um antibiótico), lítio(utilizado para tratar alguns transtornos psiquiátricos) e preparados à base de erva-de-São-João –Hypericum perforatum(planta medicinal para a depressão). Em casos muito raros, mirtazapina sozinha ou junto com estes medicamentos, pode dar origem ao chamado síndrome serotoninérgico. Alguns dos sintomas deste síndrome são: febre inexplicável, suor, palpitações, diarreia, contrações musculares (incontroláveis), arrepios, reflexos exagerados, agitação, mudanças de humor e perda de consciência. Se apresenta uma combinação destes sintomas, consulte o seu médico imediatamente.
- o antidepressivo nefazodona. Pode aumentar a quantidade de mirtazapina no sangue. Informe o seu médico se está tomando este medicamento. Poderia ser necessário diminuir a dose de mirtazapina, ou aumentá-la novamente ao deixar de tomar nefazodona.
- medicamentos para a ansiedade ou o insóniocomo as benzodiazepinas.
medicamentos para a esquizofreniacomo a olanzapina.
medicamentos para as alergiascomo a cetirizina.
medicamentos para a dor intensacomo a morfina.
Em combinação com estes medicamentos, mirtazapina pode aumentar a sonolência causada por estes medicamentos.
- medicamentos para infecções:medicamentos para infecções bacterianas (como a eritromicina), medicamentos para infecções por fungos (como o cetoconazol) e os medicamentos para o VIH/SIDA (inibidores da protease do VIH).
Se se tomam junto com mirtazapina, estes medicamentos podem aumentar a quantidade de mirtazapina no sangue. Informe o seu médico se está tomando estes medicamentos. Poderia ser necessário diminuir a dose de mirtazapina, ou aumentá-la novamente ao deixar de tomar estes medicamentos.
- medicamentos para a epilepsiacomo a carbamazepina e a fenitoína;medicamentos para a tuberculosecomo a rifampicina.
Se se tomam junto com mirtazapina, estes medicamentos podem reduzir a quantidade de mirtazapina no sangue. Informe o seu médico se está tomando estes medicamentos. Poderia ser necessário aumentar a dose de mirtazapina, ou diminuí-la novamente ao deixar de tomar estes medicamentos.
- medicamentos para prevenir a coagulação do sanguecomo a varfarina.
Mirtazapina pode aumentar os efeitos da varfarina no sangue. Informe o seu médico se está tomando este medicamento. Em caso de tomar os dois ao mesmo tempo, recomenda-se que o médico faça controles no sangue.
Toma de Mirtazapina Tarbiscom os alimentos, bebidas e álcool
Pode sentir-se sonolento se beber álcool enquanto estiver em tratamento com mirtazapina.
Recomenda-se não beber nada de álcool.
Pode tomar mirtazapina com ou sem alimentos.
Gravidez, lactação e fertilidade
Consulte o seu médico ou farmacêutico antes de utilizar qualquer medicamento.
A experiência limitada da administração de mirtazapina a mulheres grávidas não indica um aumento de risco. No entanto, deve ter-se cuidado se se usa durante a gravidez.
Se está grávida ou em período de lactação, acredita que possa estar grávida ou tem intenção de ficar grávida, consulte o seu médico ou farmacêutico antes de utilizar este medicamento. Se usa mirtazapina até, ou pouco antes, do parto, o seu filho será examinado para detectar possíveis efeitos adversos.
Se está tomando mirtazapina durante a gravidez, diga à sua parteira e/ou médico. Quando se tomam durante a gravidez medicamentos semelhantes (denominados antidepressivos inibidores da recaptura de serotonina: ISRS), pode aumentar o risco de que o bebê sofra uma doença grave chamada hipertensão pulmonar persistente do recém-nascido (HPPN), ocasionando que o niño respire mais rápido e pareça azulado. Estes sintomas geralmente começam durante as primeiras 24 horas após o nascimento. Se isso ocorrer no seu caso, deve contactar imediatamente o seu médico e/ou parteira.
Consulte o seu médico se pode dar o peito enquanto toma Mirtazapina Tarbis.
Condução e uso de máquinas
Mirtazapina Tarbis pode afetar a sua concentração ou estado de alerta.
Durante o tratamento com Mirtazapina Tarbis pode que se sinta sonolento ou mareado. Não conduza nem maneje ferramentas ou máquinas até que saiba como lhe afeta o tratamento com Mirtazapina Tarbis.
Mirtazapina Tarbis contém aspartamo
Este medicamento pode ser prejudicial para pessoas com fenilcetonúria porque contém aspartamo que é uma fonte de fenilalanina.
3. Como tomar Mirtazapina Tarbis
Siga exatamente as instruções de administração deste medicamento indicadas pelo seu médico ou farmacêutico. Em caso de dúvida, consulte novamente o seu médico ou farmacêutico.
Quanto tomar
A dose inicial normal é de 15 ou 30mg ao dia.O seu médico pode recomendar-lhe aumentar a dose após alguns dias até à quantidade que seja melhor para si (entre 15 e 45 mg ao dia). Normalmente a dose é a mesma para todas as idades. No entanto, se é uma pessoa idosa ou se padece uma doença dos rins ou do fígado, o seu médico poderia cambiar a dose.
Quando tomar
?Tome Mirtazapina Tarbis à mesma hora todos os dias.
É melhor tomar a dose de mirtazapina de uma vez antes de deitar. No entanto, o seu médico pode recomendar-lhe que divida a sua dose de mirtazapina pela manhã e pela noite antes de deitar. A dose mais alta deve ser tomada antes de deitar.
Tome o comprimido bucodispersável da seguinte forma
Os comprimidos são tomados por via oral.
- Não pressione o comprimido bucodispersável
Para evitar que o comprimido bucodispersável se esmague, não pressione o alvéolo (Figura A).
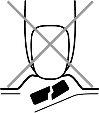
Fig. A.
- Separe um alvéolo
Cada blister contém seis alvéolos, que estão separados por perfurações. Separe um alvéolo seguindo as linhas perfuradas (Figura 1).
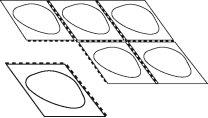
Fig. 1.
- Abrir o alvéolo
Retire cuidadosamente a lâmina, começando na esquina indicada pela seta (Figuras 2 e 3).
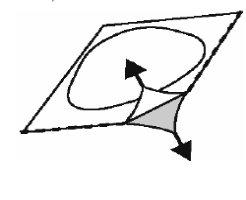
Fig. 2.
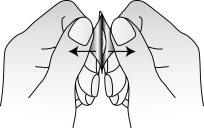
Fig. 3.
- Saia o comprimido bucodispersável
Saia o comprimido bucodispersável com as mãos secas e ponha-o na língua (Figura 4).
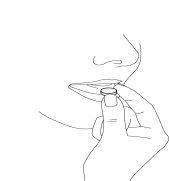
Fig. 4.
Desfar-se-á rapidamente e pode engoli-lo sem água.
Quando pode esperar encontrar-se melhor
Normalmente Mirtazapina Tarbis começará a fazer efeito após 1 ou 2 semanas e após 2 a 4 semanas poderia começar a encontrar-se melhor. É importante que durante as primeiras semanas de tratamento fale com o seu médico sobre os efeitos de mirtazapina:
?entre 2 e 4 semanas após ter começado a tomar mirtazapina, fale com o seu médico sobre como lhe afetou este medicamento.
Se ainda não se encontra melhor, o seu médico poderia prescrever-lhe uma dose maior. Nesse caso, fale novamente com o seu médico após outras 2-4 semanas.
Normalmente necessitará tomar mirtazapina até que os sintomas de depressão tenham desaparecido durante 4-6 meses.
Se tomar mais Mirtazapina Tarbis do que devia
?Se si ou alguém toma demasiada Mirtazapina Tarbis, consulte um médico imediatamente. Também pode ligar para o Serviço de Informação Toxicológica, telefone 91 562 04 20.
Os sintomas mais prováveis de uma sobredose de mirtazapina (sem outros medicamentos ou álcool) são a sonolência, desorientação e palpitações.
Se esqueceu de tomar Mirtazapina Tarbis
Se tem que tomar a sua dose uma vez ao dia
- se esqueceu de tomar a sua dose de mirtazapina, não tome a dose esquecida. Salte-a e tome a dose habitual no dia seguinte.
Se tem que tomar a sua dose duas vezes ao dia
- se esqueceu a dose da manhã, simplesmente tome-a junto com a dose da noite
- se esqueceu a dose da noite, não a tome na manhã seguinte; salte-a e continue com as suas doses normais pela manhã e pela noite.
- se esqueceu ambas as doses, não tente recuperá-las. Salte-as e no dia seguinte continue com a dose normal pela manhã e pela noite.
Se interromper o tratamento com Mirtazapina Tarbis
?Deixe de tomar mirtazapina apenas se o consultar com o seu médico.
Se o deixar demasiado cedo, a depressão poderia reaparecer. Quando se encontrar melhor, fale com o seu médico. O seu médico decidirá quando pode deixar o tratamento.
Não deixe de tomar mirtazapina bruscamente, mesmo que a depressão tenha desaparecido. Se deixar de tomar mirtazapina de forma brusca, pode sentir-se doente, mareado, agitado ou ansioso e ter dores de cabeça. Estes sintomas podem ser evitados deixando o tratamento gradualmente. O seu médico indicar-lhe-á como diminuir a dose gradualmente.
Se tiver alguma outra dúvida sobre o uso deste medicamento, pergunte ao seu médico ou farmacêutico.
4. Possíveis efeitos adversos
Tal como todos os medicamentos, Mirtazapina Tarbis pode produzir efeitos adversos, embora nem todas as pessoas os sofram.
Alguns efeitos adversos são mais prováveis do que outros. Os efeitos adversos possíveis de mirtazapina se indicam a seguir e podem dividir-se em:
- Muito frequentes:afetam mais de 1 de cada 10 doentes
- Frequentemente:afetam entre 1 e 10 de cada 100 doentes
- Pouco frequentes:afetam entre 1 e 10 de cada 1.000 doentes
- Raros:afetam entre 1 e 10 de cada 10.000 doentes
- Muito raros:afetam menos de 1 de cada 10.000 doentes
- Desconhecido:não se pode estimar da informação disponível
Muito frequentes:
- aumento do apetite e aumento de peso
- sonolência
- dor de cabeça
- boca seca
Frequentemente:
- letargia
- tontura
- trejeção
- náuseas
- diarreia
- vómitos
- prisão de ventre
- urticária ou erupções na pele (exantema)
- dores nas articulações (artralgia) ou músculos (mialgia)
- dor nas costas
- tontura ou desmaio ao levantar-se rapidamente (hipotensão ortostática)
- inchaço (normalmente nos tornozelos ou pés) devido à retenção de líquidos (edema)
- fadiga
- sonhos vívidos
- confusão
- ansiedade
- dificuldades para dormir
- problemas de memória, que na maioria dos casos se resolveram quando se suspendeu o tratamento.
Pouco frequentes:
- sentimento de euforia exagerada (mania)
?Deixe de tomar mirtazapina e consulte o seu médico imediatamente.
- sensação estranha na pele, por exemplo, queimadura, picadas, formigamento ou dormência (parestesia)
- movimentos involuntários de agitação das pernas durante o sono
- desmaios (síncope)
- sensação de adormecimento da boca (hipoestesia oral)
- baixa tensão
- pesadelos
- agitação
- alucinações
- incapacidade para manter-se quieto
Raros:
- coloração amarela dos olhos ou da pele; pode sugerir alterações no funcionamento do fígado (icterícia).
?Deixe de tomar mirtazapina e consulte o seu médico imediatamente.
- tics ou contrações musculares (mioclono)
- pancreatite
Frequência desconhecida:
- signos de infecção, tais como febre alta inexplicável e súbita, dor de garganta e úlceras na boca (agranulocitose).
?Deixe de tomar mirtazapina e consulte o seu médico imediatamente para fazer um exame de sangue.
Em casos raros, mirtazapina pode provocar alterações na produção de células sanguíneas (depressão da medula óssea). Algumas pessoas se tornam menos resistentes às infecções porque mirtazapina pode provocar uma diminuição temporária dos glóbulos brancos do sangue (granulocitopenia). Em casos raros, mirtazapina também pode provocar uma diminuição dos glóbulos vermelhos e brancos e das plaquetas (anemia aplásica), uma diminuição das plaquetas (trombocitopenia) ou um aumento no número de glóbulos brancos no sangue (eosinofilia).
- ataque epiléptico (convulsões).
?Deixe de tomar mirtazapina e consulte o seu médico imediatamente.
- combinação de sintomas, como febre inexplicável, suor, palpitações, diarreia, contrações musculares (incontroláveis), arrepios, reflexos exagerados, agitação, mudanças de humor e perda de consciência. Em casos muito raros, estes sintomas podem ser sinais de um distúrbio chamado “síndrome serotoninérgica”.
?Deixe de tomar mirtazapina e consulte o seu médico imediatamente.
- pensamentos de se fazer mal a si mesmo ou de suicídio
?Deixe de tomar mirtazapina e consulte o seu médico imediatamente.
- sensações anormais na boca (parestesia oral)
- inchaço na boca (edema bucal)
- hiponatremia
- secreção inadequada de hormona antidiurética
- aumento dos níveis de creatina quinase no sangue
- dificuldade para urinar (retenção urinária)
- dor muscular, rigidez e/ou fraqueza, escurecimento ou descoloração da urina (rabdomiólise).
- manchas vermelhas no tronco, como máculas circunscritas ou circulares, frequentemente com bolhas no centro, desprendimento da pele, úlceras na boca, na garganta, no nariz, nos genitais e nos olhos. Estes eritemas cutâneos graves podem ser precedidos de febre e sintomas gripais (síndrome de Stevens-Johnson, necrólise epidérmica tóxica) ?Deixe de tomar mirtazapina e consulte o seu médico imediatamente.
- eritema generalizado, temperatura corporal elevada e aumento do tamanho dos gânglios linfáticos (síndrome DRESS ou síndrome de hipersensibilidade medicamentosa) ?Deixe de tomar mirtazapina e consulte o seu médico imediatamente.
Se experimentar efeitos adversos, consulte o seu médico ou farmacêutico, mesmo que se trate de efeitos adversos que não aparecem neste prospecto.
Comunicação de efeitos adversos:
Se experimentar qualquer tipo de efeito adverso, consulte o seu médico ou farmacêutico, mesmo que se trate de possíveis efeitos adversos que não aparecem neste prospecto. Também pode comunicá-los diretamente através do Sistema Espanhol de Farmacovigilância de medicamentos de Uso Humano: https://www.notificaram.es. Mediante a comunicação de efeitos adversos, você pode contribuir para proporcionar mais informações sobre a segurança deste medicamento.
5. Conservação de Mirtazapina Tarbis
Mantenha este medicamento fora da vista e do alcance das crianças.
Não utilize este medicamento após a data de validade que aparece no envase e no blister após CAD. A data de validade é o último dia do mês que se indica.
Não conserve a uma temperatura superior a 25ºC.
Conservar no envase original para protegê-lo da luz e da humidade.
Os medicamentos não devem ser jogados pelos desgotos nem na lixeira. Deposite os envases e os medicamentos que não precisa no Ponto SIGRE da farmácia. Em caso de dúvida, pergunte ao seu farmacêutico como se livrar dos envases e dos medicamentos que não precisa. Dessa forma, ajudará a proteger o meio ambiente.
6. Conteúdo do envase e informação adicional
Composição de Mirtazapina Tarbis
- O princípio ativo é mirtazapina.
Mirtazapina Tarbis 30 mg comprimidos bucodispersáveis contém 30 mg de mirtazapina por comprimido.
- Os demais componentes são: manitol, celulose microcristalina, carbonato magnésico, hidroxipropilcelulose de baixa substituição, sílica coloidal anidra, L-metionina, crospovidona, celulose microcristalina e goma guar (Avicel CE-15), aspartamo (E951), aroma de laranja silesia e estearato magnésico.
Aspecto do produto e conteúdo do envase
Mirtazapina Tarbis 30 mg comprimidos bucodispersáveis são de cor branca, redondos, biconvexos e marcados com ´M2` em uma face.
Os comprimidos bucodispersáveis são envasados em blisters à prova de crianças, perfurados para dose unitária.
Estão disponíveis os seguintes tamanhos de envase: 30 comprimidos bucodispersáveis.
Titular da autorização de comercialização e responsável pela fabricação
Titular da autorização de comercialização:
TARBIS FARMA, S.L.
Gran Vía Carlos III, 94
08028-Barcelona (Espanha)
Responsável pela fabricação
Actavis HF
Reyjavikuvegur 78
220 Hafnarfjordur
Islândia
Ó
Actavis Limited
BLB 016 Bulebel Industrial Estate
Zejtun ZTN 3000
Malta
Data da última revisão deste prospecto:Outubro 2020
A informação detalhada e atualizada deste medicamento está disponível na página Web da Agência Espanhola de Medicamentos e Produtos Sanitários (AEMPS)http://www.aemps.gob.es/

Quanto custa o MIRTAZAPINA TARBIS 30 mg COMPRIMIDOS ORODISPERSÍVEIS em Espanha em 2025?
O preço médio do MIRTAZAPINA TARBIS 30 mg COMPRIMIDOS ORODISPERSÍVEIS em dezembro de 2025 é de cerca de 17.05 EUR. Os valores podem variar consoante a região, a farmácia e a necessidade de receita. Confirme sempre com uma farmácia local ou fonte online para obter informações atualizadas.
- País de registo
- Preço médio em farmácia17.05 EUR
- Substância ativa
- Requer receita médicaSim
- Fabricante
- Esta informação é apenas para referência e não constitui aconselhamento médico. Consulte sempre um médico antes de tomar qualquer medicamento. A Oladoctor não se responsabiliza por decisões médicas baseadas neste conteúdo.
- Alternativas a MIRTAZAPINA TARBIS 30 mg COMPRIMIDOS ORODISPERSÍVEISForma farmacêutica: COMPRIMIDO, 15 mgSubstância ativa: mirtazapineFabricante: Laboratorios Alter S.A.Requer receita médicaForma farmacêutica: COMPRIMIDO, 30 mgSubstância ativa: mirtazapineFabricante: Laboratorios Alter S.A.Requer receita médicaForma farmacêutica: COMPRIMIDO, 15 mgSubstância ativa: mirtazapineFabricante: Almus Farmaceutica S.A.U.Requer receita médica
Alternativas a MIRTAZAPINA TARBIS 30 mg COMPRIMIDOS ORODISPERSÍVEIS noutros países
As melhores alternativas com o mesmo princípio ativo e efeito terapêutico.
Alternativa a MIRTAZAPINA TARBIS 30 mg COMPRIMIDOS ORODISPERSÍVEIS em Polónia
Alternativa a MIRTAZAPINA TARBIS 30 mg COMPRIMIDOS ORODISPERSÍVEIS em Ukraine
Médicos online para MIRTAZAPINA TARBIS 30 mg COMPRIMIDOS ORODISPERSÍVEIS
Avaliação de posologia, efeitos secundários, interações, contraindicações e renovação da receita de MIRTAZAPINA TARBIS 30 mg COMPRIMIDOS ORODISPERSÍVEIS – sujeita a avaliação médica e regras locais.









