LEUPRORELINA GP-PHARM DEPOT TRIMESTRAL 22,5 mg PÓ E SOLVENTE PARA SUSPENSÃO INJETÁVEL DE LIBERTAÇÃO PROLONGADA

Pergunte a um médico sobre a prescrição de LEUPRORELINA GP-PHARM DEPOT TRIMESTRAL 22,5 mg PÓ E SOLVENTE PARA SUSPENSÃO INJETÁVEL DE LIBERTAÇÃO PROLONGADA

Como usar LEUPRORELINA GP-PHARM DEPOT TRIMESTRAL 22,5 mg PÓ E SOLVENTE PARA SUSPENSÃO INJETÁVEL DE LIBERTAÇÃO PROLONGADA
Introdução
Prospecto: informação para o utilizador
Leuprorelina GP-Pharm Depot Trimestral 22,5 mg pó e dissolvente para suspensão de libertação prolongada injectável
Acetato de leuprorelina
Leia todo o prospecto atentamente antes de começar a usar este medicamento, porque contém informações importantes para si.
- Conserva este prospecto, porque pode ter que voltar a lê-lo.
- Se tiver alguma dúvida, consulte o seu médico ou farmacêutico.
- Este medicamento foi-lhe prescrito apenas para si, e não deve dá-lo a outras pessoas, embora tenham os mesmos sintomas que si, porque pode prejudicá-las.
- Se experimentar efeitos adversos, consulte o seu médico ou farmacêutico, mesmo que se trate de efeitos adversos que não aparecem neste prospecto. Ver secção 4.
Conteúdo do prospecto
- O que é Leuprorelina GP-Pharm Depot Trimestral e para que é utilizado
- O que necessita de saber antes de começar a usar Leuprorelina GP-Pharm Depot Trimestral
- Como usar Leuprorelina GP-Pharm Depot Trimestral
- Possíveis efeitos adversos
- Conservação de Leuprorelina GP-Pharm Depot Trimestral
- Conteúdo do envase e informação adicional
1. O que é Leuprorelina GP-Pharm Depot Trimestral e para que é utilizado
Leuprorelina GP-Pharm Depot Trimestral é um frasco que contém um pó branco, que se reconstitui em forma de suspensão para injeção num músculo. Este medicamento contém o princípio ativo leuprorelina (também conhecido como leuprolide), que pertence a um grupo de medicamentos chamado agonistas da hormona liberadora de gonadotropina (LHRH), que são medicamentos que reduzem a testosterona (uma hormona sexual).
O seu médico prescreveu-lhe este medicamento para o tratamento paliativo do cancro da próstata avançado.
2. O que necessita de saber antes de começar a usar Leuprorelina GP-Pharm Depot Trimestral
Não use Leuprorelina GP-Pharm Depot Trimestral:
- Se é alérgico (hipersensível) à LHRH, aos agonistas da LHRH ou a qualquer um dos outros componentes deste medicamento (incluídos na secção 6). Uma reação alérgica pode manifestar-se como erupção cutânea, picazón, dificuldade para respirar ou inchação da face, lábios, garganta ou língua.
- Se se submeteu a uma orquiectomia (reseção dos testículos).
- Se é uma mulher ou uma criança.
- Este medicamento não deve ser utilizado sozinho (em monoterapia) para o tratamento do cancro da próstata quando a medula espinal está comprimida ou o cancro se espalhou até à medula.
Advertências e precauções
- Consulte o seu médico ou farmacêutico antes de começar a tomar este medicamento.
- É possível que a sua doença piore durante as primeiras semanas do tratamento, mas deve melhorar com o tratamento contínuo. Os sinais e sintomas incluem: aumento temporário da testosterona (hormona masculina), sofocos, dor óssea, distúrbios do sistema nervoso (incluindo depressão) ou obstrução urinária.
- Se acredita que está a experimentar uma reação alérgica (falta de ar, asma, rinite, inchação da face, urticária, erupção cutânea), pare de tomar este medicamento e informe o seu médico.
- Informa o seu médico se tem risco de sofrer, ou sofre, alguma das seguintes doenças, porque pode necessitar de revisões mais frequentes:
- Hematomas ou sangramento sem explicação ou se experimenta mal-estar geral. Embora seja raro, estes podem ser sintomas de alterações no número de glóbulos vermelhos ou brancos
- Doença metabólica
- Problemas cardíacos, ou batimento cardíaco palpitante
- Diabetes.
- O médico deve ser informado de qualquer antecedente clínico pessoal de adenoma hipofisário (tumor não maligno da hipófise). Foram descritos casos de apoplexia hipofisária (perda parcial de tecido da hipófise) após a administração inicial deste tipo de medicamento a pacientes com adenoma hipofisário. Pode manifestar-se apoplexia hipofisária, sob a forma de dor de cabeça repentina, meningismo, distúrbios da visão ou visão alterada, incluindo cegueira, e ocasionalmente uma diminuição do nível de consciência.
- O seu médico deve saber se sofre de um distúrbio da coagulação, trombocitopenia ou se está em tratamento com anticoagulantes. É possível que a sua função hepática deva ser supervisionada, porque foram descritas alterações do fígado e icterícia (coloração amarelada dos olhos e da pele) com a administração de leuprorelina.
- Foram descritos fractura da coluna, paralisia, pressão arterial baixa e pressão arterial alta com o tratamento com leuprorelina.
- Foram notificados casos de depressão em pacientes em tratamento com este medicamento, que pode ser grave. Se está a usar este medicamento e se sente deprimido, informe o seu médico.
- Foram descritas redução da densidade óssea (ossos frágeis ou mais finos) após a administração de leuprorelina. O médico pode considerar a possibilidade de adicionar um antiandrógeno ao tratamento com este medicamento. Neste caso, o médico estará alerta para detectar a presença de inflamação das veias (tromboflebite) e outros sinais de distúrbios da coagulação e edema (inchação das mãos, pés ou tornozelos) que têm mais risco de ocorrer quando se adiciona tratamento antiandrogénico a este medicamento.
- Informa o seu médico se sente pressão na medula espinal e/ou apresenta distúrbios urinários e/ou hematúria (sangue na urina); nesse caso, o médico irá discutir a necessidade de tratamentos adicionais para prevenir complicações neurológicas (por exemplo, formigamento nas mãos e pés, paralisia) ou obstrução da uretra (canal que conecta a bexiga com o exterior do corpo). Será supervisionado estreitamente durante as primeiras semanas de tratamento.
- Os pacientes podem experimentar alterações metabólicas (por exemplo, intolerância à glicose ou agravamento da diabetes existente), alterações de peso e distúrbios cardiovasculares.
- Os pacientes com doença metabólica ou cardiovascular, e especialmente os pacientes com antecedentes de insuficiência cardíaca congestiva (doença em que o coração já não pode bombear suficiente sangue para o resto do corpo), devem ser controlados durante o tratamento com leuprorelina.
- Consulte o seu médico, farmacêutico ou enfermeiro se tem fígado gordo.
- Durante o tratamento, devem ser realizadas algumas análises de sangue para comprovar se este medicamento é eficaz.
- Pode experimentar uma perda de interesse nas relações sexuais, sofocos e ocasionalmente pode produzir-se uma redução no tamanho e na função dos testículos.
- Pode voltar a ser fértil novamente quando se interromper o tratamento com este medicamento.
- Este medicamento pode interferir com certos exames analíticos, por isso deve assegurar-se de que o seu médico sabe que está a usar este medicamento.
- Podem produzir-se convulsões em pacientes predispostos (pacientes com histórico de convulsões, epilepsia, distúrbios cerebrovasculares, anomalias ou tumores do sistema nervoso central), em pacientes que tomam fármacos que podem causar convulsões e, em menor medida, em pacientes que não apresentam estas características.
- Informa o seu médico se padece alguma afecção cardíaca ou dos vasos sanguíneos ou está a ser tratado para isso, incluindo medicamentos para controlar o ritmo cardíaco (arritmias). O risco de problemas do ritmo cardíaco pode aumentar quando se utiliza este medicamento.
- Entre em contacto com o seu médico imediatamente se padece cefaleias intensas ou recorrentes, problemas visuais e acúfenos ou zumbidos.
- Foram comunicados erupções cutâneas graves, incluindo síndrome de Stevens-Johnson/necrólise epidérmica tóxica (SJS/NET) em associação com leuprorelina. Suspenda o uso de leuprorelina e procure atendimento médico imediatamente se nota algum dos sintomas relacionados com estas reações cutâneas graves descritas na secção 4.
Uso de Leuprorelina GP-Pharm Depot Trimestral com outros medicamentos
Informa o seu médico ou farmacêutico se está a utilizar, utilizou recentemente ou pode ter que utilizar qualquer outro medicamento. É possível que continue a ser adequado o tratamento com este medicamento; o médico poderá decidir o que é adequado para si.
Este medicamento pode interferir com alguns medicamentos utilizados para tratar problemas do ritmo cardíaco (por exemplo: quinidina, procainamida, amiodarona e sotalol) ou pode aumentar o risco de problemas do ritmo cardíaco quando se utiliza com outros medicamentos (por exemplo: metadona (utilizada para o alívio da dor e para a desintoxicação de outros medicamentos), moxifloxacino (um antibiótico), antipsicóticos usados para tratar doenças mentais graves).
Gravidez e lactação
O uso deste medicamento não é indicado em mulheres.
Este medicamento é contraindicado durante a gravidez. Podem produzir-se abortos espontâneos se este medicamento for administrado durante a gravidez.
Condução e uso de máquinas
Não há estudos específicos sobre os efeitos deste medicamento sobre a capacidade para conduzir e utilizar máquinas.
Podem produzir-se alterações da visão e tonturas durante o tratamento. Se se vê afectado, não conduza nem maneje máquinas.
Leuprorelina GP-Pharm DepotTrimestralcontémmenos de 23 mg de sódio (1 mmol) por dose; isto é, essencialmente “isento de sódio”.
3. Como usar Leuprorelina GP-Pharm Depot Trimestral.
Posologia
Este medicamento só deve ser administrado pelo seu médico ou enfermeiro. Eles serão os responsáveis pela preparação do produto.
Adultos e idosos:
A dose recomendada deste medicamento é de uma injeção cada três meses. O pó é reconstituído para formar uma suspensão que é administrada em forma de injeção intramuscular (num músculo) uma vez cada três meses.
O local de injeção deve ser alterado a intervalos regulares.
Este medicamento deve ser administrado unicamente por via intramuscular. Não deve ser administrado por outra via.
A pauta do tratamento será decidida pelo seu médico.
Uso em crianças:Este medicamento não é indicado em crianças.
Se usa mais Leuprorelina GP-Pharm Depot Trimestral do que deve
Isso é improvável, porque o médico ou a enfermeira saberão qual é a dose adequada. No entanto, se suspeita que recebeu mais medicamento do que devia, informe o seu médico imediatamente para que possam ser tomadas as medidas necessárias.
Em caso de sobredose ou ingestão acidental, consulte o Serviço de Informação Toxicológica, tel: 91 562 04 20, indicando o medicamento e a quantidade usada.
Se esqueceu de usar Leuprorelina GP-Pharm Depot Trimestral
É importante que não salte uma dose deste medicamento. Assim que saiba que saltou uma injeção, entre em contacto com o seu médico, que lhe administrará a próxima injeção.
Se interromper o tratamento com Leuprorelina GP-Pharm Depot Trimestral
Como o tratamento médico implica a administração deste medicamento durante um longo período de tempo, ao interromper o tratamento pode experimentar-se um agravamento dos sintomas relacionados com a doença. Por isso, não deve interromper o tratamento de forma prematura sem o consentimento do seu médico.
Se tiver alguma outra dúvida sobre o uso deste medicamento, pergunte ao seu médico ou farmacêutico.
4. Possíveis efeitos adversos
Como todos os medicamentos, este medicamento pode produzir efeitos adversos, embora nem todas as pessoas os sofram.
Informa o seu médico imediatamente se nota algum dos seguintes sintomas:
- sofre respiração sibilante, dificuldade para respirar, inchação dos párpados, face ou lábios, erupção cutânea ou picazón (especialmente se o afeta a todo o corpo) de forma repentina.
- Frequência não conhecida (a frequência não pode ser estimada a partir dos dados disponíveis):
- Se nota que no tronco tem manchas circulares ou em forma de alvo, de cor vermelha e não elevadas, frequentemente com bolhas centrais, descamação da pele, úlceras na boca, na garganta, no nariz, nos genitais e nos olhos. Estas erupções cutâneas graves podem estar precedidas de febre e sintomas de tipo gripal (síndrome de Stevens-Johnson/necrólise epidérmica tóxica).
- Vermelhidão da pele e erupção com picazón (erupção cutânea tóxica).
- Uma reação cutânea que provoca granos ou manchas vermelhas na pele, que podem parecer um alvo, com um centro vermelho escuro rodeado de anéis de cor vermelha mais clara (eritema multiforme).
Foram descritos os seguintes efeitos adversos:
Muito frequentes (podem afetar mais de 1 em cada 10 pessoas):
Sofocos e reações no local de administração.
Frequentes (podem afetar até 1 em cada 10 pessoas):
Sudores frios, hiperhidrose (aumento da sudorese), picazón, fadiga, insónia, diminuição do desejo sexual, vertigem, rubor, sensação de tontura (náuseas), diarreia, diminuição do apetite, disfunção eréctil, astenia (falta ou perda de força), dor nos ossos, dor nas articulações e reações no local da injeção, tais como dor, irritação, eritema (vermelhidão da pele). Dor do tracto urinário, fluxo urinário diminuído, necessidade de urinar com frequência, alterações de humor e depressão em tratamentos prolongados com leuprorelina, alteração das enzimas hepáticas, hiperlipidemia (níveis elevados de lípidos no sangue), aumento de açúcar no sangue.
Pouco frequentes (podem afetar até 1 em cada 100 pessoas):
Colesterol elevado, distúrbios do sono, inquietude, alteração do gosto, formigamento (alteração na sensibilidade da pele), dor de cabeça, letargia (sonolência), visão borrosa, pleurisia, zumbido nos ouvidos (tinnitus), dor no abdómen superior, constipação, pápula, erupção, picazón generalizado, sudores noturnos, dor nas costas, dor muscular, dor no pescoço, dor nas mamas, dor pélvica, atrofia testicular, distúrbio testicular, sensação de calor, alterações de humor e depressão em tratamentos a curto prazo com leuprorelina. Alterações nos valores sanguíneos e no electrocardiograma ECG (prolongamento do intervalo QT). Reações no local da injeção, tais como urticária, calor e hemorragia.
Não conhecida (a frequência não pode ser estimada a partir dos dados disponíveis)
Inflamação dos pulmões, doença pulmonar
Hipertensão intracraniana idiopática (aumento da pressão intracraniana ao redor do cérebro caracterizado por cefaleias, diplopia e outros sintomas visuais, acúfenos ou zumbidos em um ou ambos os ouvidos).
Comunicação de efeitos adversos:
Se experimenta algum tipo de efeito adverso, consulte o seu médico ou farmacêutico, mesmo que se trate de efeitos adversos que não aparecem neste prospecto. Também pode comunicá-los directamente através do Sistema Espanhol de Farmacovigilância de Medicamentos de Uso Humano: www.notificaRAM.es. Mediante a comunicação de efeitos adversos, pode contribuir para proporcionar mais informações sobre a segurança deste medicamento.
5. Conservação de Leuprorelina GP-Pharm Depot Trimestral
O seu médico ou farmacêutico saberão como conservar este medicamento.
Mantenha este medicamento fora da vista e do alcance das crianças.
Não conserve a uma temperatura superior a 25ºC. Não congele.
Não utilize este medicamento após a data de validade que aparece no envase, frasco e seringa após “CAD”. A seringa tem a mesma validade que o frasco.
A data de validade é o último dia do mês que se indica.
Os medicamentos não devem ser deitados fora pelas águas residuais nem pelo lixo. Deposite os envases e os medicamentos que não precisa no Ponto SIGRE da farmácia. Pergunte ao seu farmacêutico como se livrar dos envases e dos medicamentos que não precisa. Dessa forma, ajudará a proteger o meio ambiente.
6. Conteúdo do envase e informação adicional
Composição de Leuprorelina GP-Pharm Depot Trimestral
O princípio ativo é acetato de leuprorelina. Cada frasco contém 22,5 mg de acetato de leuprorelina.
A concentração do produto reconstituído é de 11,25 mg/ml. Os demais componentes são: polissorbato 80, manitol (E-421), carmelosa sódica (E-466), trietil citrato e poli(ácido láctico) (PLA).
O solvente contém (seringa pré-carregada): manitol, água para preparações injetáveis, hidróxido de sódio (para ajuste de pH) e ácido clorídrico (para ajuste de pH).
Aspecto do produto e conteúdo do envase
Cada envase contém um frasco com 22,5 mg de acetato de leuprorelina, uma seringa pré-carregada com 2 ml de solvente, um sistema adaptador e uma agulha estéril do calibre 20.
Titular da autorização de comercialização e responsável pela fabricação
GP-PHARM, S.A.
Pol. Ind. Els Vinyets – Els Fogars Sector 2
Carretera comarcal 244, km22
08777 Sant Quintí de Mediona
Espanha
Este medicamento está autorizado nos estados membros do Espaço Económico Europeu com os seguintes nomes:
Espanha: Leuprorelina GP-Pharm Depot Trimestral 22,5 mg pó e solvente para suspensão de libertação prolongada injetável
Alemanha: Lutrate Depot 22,5 mg Pulver und Lösungsmittel zur Herstellung einer Depot-Injektionssuspension
Portugal: Lutrate Depot 22,5 mg / 2 ml pó e veículo para suspensão injectável de libertação prolongada
Grécia: Lutrate Depot 22,5mg Κ?νις και διαλ?της για παρασκευ? ενεσ?μου εναιωρ?ματος παρατεταμ?νης αποδ?σμευσης
Itália: Politrate
Hungria: Politrate Depot 22,5 mg
Áustria: Lutrate 3-Monats-Depot 22,5 mg Pulver und Lösungsmittel zur Herstellung einer Depot-injektionssuspension
República Checa: Lutrate Depot 22,5mg
Polônia: Lutrate Depot
Bulgária: ?????? ???? 22,5 mg ???? ? ??????????? ?? ??????????? ????????? ? ???????? ?????????????
Data da última revisão deste prospecto:Abril 2025.
A informação detalhada e actualizada deste medicamento está disponível na página Web da Agência Espanhola de Medicamentos e Produtos Sanitários (AEMPS) http://www.aemps.gob.es/.
Esta informação está destinada unicamente a profissionais do sector sanitário.
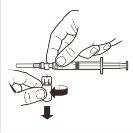
Como preparar a injeção?
IMPORTANTE: Leia detenidamente antes de administrar o produto (as Instruções de uso também se incluem na bandeja que contém os componentes do kit).
Deverá seguir-se uma técnica asséptica durante o procedimento de reconstituição.
Utilize unicamente o solvente incluído no kit comercial.
Uma vez misturado, o produto deve ser administrado imediatamente por injeção intramuscular única.
Este medicamento é de um só uso. Qualquer resto de suspensão deve ser descartado.
Verifique o conteúdo do kit e certifique-se de que inclui tudo o mencionado no prospecto.
O envase contém:
1 (um) frasco de Leuprorelina GP-Pharm Depot 22,5 mg (acetato de leuprorelina) pó para suspensão injetável
1 (uma) seringa pré-carregada que contém o solvente para a suspensão (solução injetável de manitol 0,8%)
1 (um) dispositivo para a reconstituição estéril de um só uso, incluindo 1 (uma) agulha estéril.
1 |
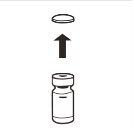
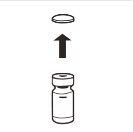
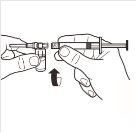
| Retire completamente a tampa de fechamento a pressão da parte superior do frasco, de modo que o tampão de borracha fique exposto. Confirme que não restam partes da tampa de fechamento a pressão no frasco. |
2 |
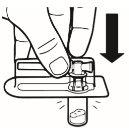
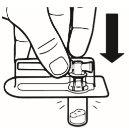
| Coloque o frasco em posição vertical sobre uma mesa. Retire a cobertura do blíster que contém o adaptador do frasco (MIXJECT). Não retire o adaptador do frasco do blíster.Coloque firmemente o blíster que contém o adaptador do frasco na parte superior do frasco, perfurando o tampãoem posição totalmente vertical. Pressione suavemente para baixoaté que note que encaixa em sua posição. |
3 |
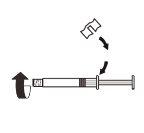
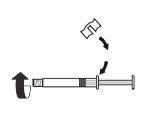
| Fixe a peça branca à seringa até que note que encaixa. Desenrosqueo tampão rígido da seringa no sentido anti-horário. Depois, retire o blíster do sistema adaptador MIXJECT. |
4 |
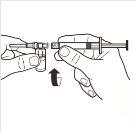
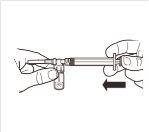
| Conecte a seringa ao sistema adaptador enroscando-a no sentido horário na abertura lateral do sistema adaptador. Para assegurar uma conexão hermética, enrosque suavemente a seringa até que se detenha. |
5 |
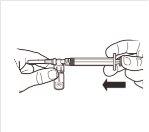
| Enquanto mantém a seringa e o frasco firmemente unidos em posição vertical, empurre lentamente o êmbolo da seringa para transferir todo o solvente para o frasco. |
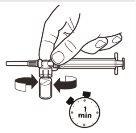
6 |
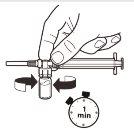
| Com a seringa ainda unida ao frasco, agite suavemente o frasco durante um minuto aproximadamenteaté obter uma suspensão leitosa uniforme.Para evitar a separação da suspensão, realize os seguintes passos sem parar. |
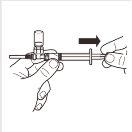
7 |
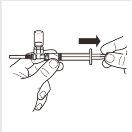
| Gire o sistema adaptador MIXJECT para que o frasco se encontre na parte superior. Segure firmemente o sistema adaptador MIXJECT pela seringa e puxe lentamente do êmbolo para transferir o conteúdo do frasco para a seringa. Parte do produto pode acumular-se ou ficar depositado na parede do frasco. Isso é normal. |
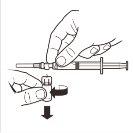
8 |
| Desconecte a seringa do sistema adaptador MIXJECT. Para isso, segure firmemente a seringa e gire o frasco no sentido horário (segurando pelo tampão de plástico do sistema adaptador). |
9 |
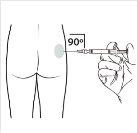
| Mantenha a seringa EM POSIÇÃO VERTICAL. Com a mão contrária, retire o protetor da agulha puxando para cima. Pressione um pouco o êmbolo para expulsar o ar da seringa. A seringa contendo o produtoestá preparada para sua administração imediata. |
10 |
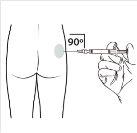
| Administre a injeção intramuscular inserindo a agulha em um ângulo de 90 graus no glúteo. Certifique-sede que a totalidade do produto seja injetada.As zonas de injeção deveriam ser alternadas. |
Instruções de uso
Para incluir na tampa da bandeja que contém os componentes do Kit do Medicamento
Leuprorelina GP-PharmDepot –Instruções de uso
Ler detenidamente antes de administrar o produto
Reconstitua imediatamente antes de administrar por injeção intramuscular única
Utilize unicamente o solvente incluído no kit comercial.
Produto destinado a uma única injeção.
Qualquer resto de suspensão deve ser descartado.
1 |
| Retire completamente a tampa de fechamento a pressão da parte superior do frasco, de modo que o tampão de borracha fique exposto. Confirme que não restam partes da tampa de fechamento a pressão no frasco. |
2 |
| Coloque o frasco em posição vertical sobre uma mesa. Retire a cobertura do blíster que contém o adaptador do frasco (MIXJECT). Não retire o adaptador do frasco do blíster.Coloque firmemente o blíster que contém o adaptador do frasco na parte superior do frasco, perfurando o tampãoem posição totalmente vertical. Pressione suavemente para baixoaté que note que encaixa em sua posição. |
3 |
| Fixe a peça branca à seringa até que note que encaixa. Desenrosqueo tampão rígido da seringa no sentido anti-horário. Depois, retire o blíster do sistema adaptador MIXJECT. |
4 |
| Conecte a seringa ao sistema adaptador enroscando-a no sentido horário na abertura lateral do sistema adaptador. Para assegurar uma conexão hermética, enrosque suavemente a seringa até que se detenha. |
5 |
| Enquanto mantém a seringa e o frasco firmemente unidos em posição vertical, empurre lentamente o êmbolo da seringa para transferir todo o solvente para o frasco. |
6 |
| Com a seringa ainda unida ao frasco, agite suavemente o frasco durante um minuto aproximadamenteaté obter uma suspensão leitosa uniforme.Para evitar a separação da suspensão, realize os seguintes passos sem parar. |
7 |
| Gire o sistema adaptador MIXJECT para que o frasco se encontre na parte superior. Segure firmemente o sistema adaptador MIXJECT pela seringa e puxe lentamente do êmbolo para transferir o conteúdo do frasco para a seringa. Parte do produto pode acumular-se ou ficar depositado na parede do frasco. Isso é normal. |
8 |
| Desconecte a seringa do sistema adaptador MIXJECT. Para isso, segure firmemente a seringa e gire o frasco no sentido horário (segurando pelo tampão de plástico do sistema adaptador). |
9 |
| Mantenha a seringa EM POSIÇÃO VERTICAL. Com a mão contrária, retire o protetor da agulha puxando para cima. Pressione um pouco o êmbolo para expulsar o ar da seringa. A seringa contendo o produtoestá preparada para sua administração imediata. |
10 |
| Administre a injeção intramuscular inserindo a agulha em um ângulo de 90 graus no glúteo. Certifique-sede que a totalidade do produto seja injetada.As zonas de injeção deveriam ser alternadas. |

Quanto custa o LEUPRORELINA GP-PHARM DEPOT TRIMESTRAL 22,5 mg PÓ E SOLVENTE PARA SUSPENSÃO INJETÁVEL DE LIBERTAÇÃO PROLONGADA em Espanha em 2025?
O preço médio do LEUPRORELINA GP-PHARM DEPOT TRIMESTRAL 22,5 mg PÓ E SOLVENTE PARA SUSPENSÃO INJETÁVEL DE LIBERTAÇÃO PROLONGADA em dezembro de 2025 é de cerca de 301.02 EUR. Os valores podem variar consoante a região, a farmácia e a necessidade de receita. Confirme sempre com uma farmácia local ou fonte online para obter informações atualizadas.
- País de registo
- Preço médio em farmácia301.02 EUR
- Substância ativa
- Requer receita médicaSim
- Fabricante
- Esta informação é apenas para referência e não constitui aconselhamento médico. Consulte sempre um médico antes de tomar qualquer medicamento. A Oladoctor não se responsabiliza por decisões médicas baseadas neste conteúdo.
- Alternativas a LEUPRORELINA GP-PHARM DEPOT TRIMESTRAL 22,5 mg PÓ E SOLVENTE PARA SUSPENSÃO INJETÁVEL DE LIBERTAÇÃO PROLONGADAForma farmacêutica: INJETÁVEL, 42 mgSubstância ativa: leuprorelinFabricante: Accord Healthcare S.L.U.Requer receita médicaForma farmacêutica: INJETÁVEL, 45 mgSubstância ativa: leuprorelinRequer receita médicaForma farmacêutica: INJETÁVEL, 22,5 mgSubstância ativa: leuprorelinRequer receita médica
Alternativas a LEUPRORELINA GP-PHARM DEPOT TRIMESTRAL 22,5 mg PÓ E SOLVENTE PARA SUSPENSÃO INJETÁVEL DE LIBERTAÇÃO PROLONGADA noutros países
As melhores alternativas com o mesmo princípio ativo e efeito terapêutico.
Alternativa a LEUPRORELINA GP-PHARM DEPOT TRIMESTRAL 22,5 mg PÓ E SOLVENTE PARA SUSPENSÃO INJETÁVEL DE LIBERTAÇÃO PROLONGADA em Polónia
Alternativa a LEUPRORELINA GP-PHARM DEPOT TRIMESTRAL 22,5 mg PÓ E SOLVENTE PARA SUSPENSÃO INJETÁVEL DE LIBERTAÇÃO PROLONGADA em Ukraine
Médicos online para LEUPRORELINA GP-PHARM DEPOT TRIMESTRAL 22,5 mg PÓ E SOLVENTE PARA SUSPENSÃO INJETÁVEL DE LIBERTAÇÃO PROLONGADA
Avaliação de posologia, efeitos secundários, interações, contraindicações e renovação da receita de LEUPRORELINA GP-PHARM DEPOT TRIMESTRAL 22,5 mg PÓ E SOLVENTE PARA SUSPENSÃO INJETÁVEL DE LIBERTAÇÃO PROLONGADA – sujeita a avaliação médica e regras locais.