
IMIGRAN 6 mg solução injetável

Pergunte a um médico sobre a prescrição de IMIGRAN 6 mg solução injetável

Como usar IMIGRAN 6 mg solução injetável
Introdução
Prospecto: informação para o utilizador
Imigran 6 mg solução injectável
Sumatriptano (como sumatriptano succinato)
Leia todo o prospecto detenidamente antes de começar a tomar este medicamento, porque contém informações importantes para si.
- Conserva este prospecto, porque pode ter que voltar a lê-lo.
- Se tiver alguma dúvida, consulte o seu médico ou farmacêutico.
- Este medicamento foi-lhe prescrito apenas para si, e não deve dá-lo a outras pessoas, embora tenham os mesmos sintomas que si, porque pode prejudicá-las.
- Se experimentar efeitos adversos, consulte o seu médico ou farmacêutico, mesmo que se trate de efeitos adversos que não aparecem neste prospecto. Ver secção 4.
Conteúdo do prospecto
- O que é Imigran e para que é utilizado
- O que precisa saber antes de começar a usar Imigran
- Como usar Imigran
- Posíveis efeitos adversos
- Conservação de Imigran
- Conteúdo do envase e informações adicionais
1. O que é Imigran e para que é utilizado
Imigran contém o princípio ativo sumatriptano que pertence ao grupo de medicamentos denominados triptanos (também conhecidos como agonistas do receptor 5-HT1).
Imigran é utilizado para aliviar a dor de cabeça e outros sintomas de um ataque de enxaqueca ou de um ataque de cefaleia em salvas.
Considera-se que a dilatação dos vasos sanguíneos do crânio é causa da dor de cabeça que aparece na enxaqueca. Este medicamento contrai os vasos sanguíneos do crânio e alivia os sintomas da enxaqueca.
2. O que precisa saber antes de começar a usar Imigran
Nãouse Imigran
- se é alérgicoao sumatriptano ou a algum dos outros componentes deste medicamento (incluídos na secção 6)
- se tem problemas de coraçãotais como estreitamento das artérias (doença isquémica coronária)ou dor de peito (angina), ou sofreu um ataque cardíaco
- se tem problemas de circulação nas pernasque lhe causam dores, como cãibras quando caminha (doença vascular periférica)
- se sofreu um acidente vascular cerebralou um mini-acidente vascular cerebral (também chamado de ataque isquémico transitório ou AIT)
- se tem a pressão sanguínea alta. Pode usar Imigran se a sua pressão sanguínea for ligeiramente elevada e estiver a ser tratada
- se padece uma doença do fígado,consulte o seu médico. Se a doença hepática for grave, Imigran não é adequado para si
- com medicamentos antimigrañosos,incluyendo aqueles que contêm ergotamina, ou medicamentos semelhantes como metisergida ou qualquer triptano/agonista do receptor 5-HT1 (medicamentos utilizados também para o tratamento da enxaqueca)
- com antidepressivos denominados IMAOs(inibidores da monoamino oxidase),ou se tomou estes medicamentos nas últimas duas semanas.
Se algum destes casos se aplica a si
?Informa ao seu médico, e não utilize Imigran.
Advertências e precauções
Consulte o seu médico ou farmacêutico antes de começar a usar Imigran.
Antes de tomar Imigran, o seu médico precisa saber se você tem algum fator de risco extra para sofrer de uma doença cardíaca:
- se é homem com mais de 40 anos
- se é mulher que iniciou a menopausa
- se fuma ou tem excesso de peso
- se tem diabetes ou o colesterol elevado
- se tem um histórico familiar de doença cardíaca.
Em muito raras ocasiões, as pessoas desenvolveram graves problemas de coração após utilizar Imigran, apesar de não apresentarem sinais de doença cardíaca previamente. Se algum dos pontos anteriores lhe acontece, pode significar que há um maior risco de desenvolver doença cardíaca, assim
?Informa ao seu médico para comprovar a sua função cardíacaantes de que lhe seja prescrito Imigran.
Se tem um histórico de ataques(convulsões)
Ou se apresenta outras condições que podem tornar mais provável que você tenha convulsões – por exemplo, uma lesão na cabeça ou alcoolismo.
?Informa ao seu médico para ser supervisionado mais de perto.
Se tem a pressão sanguínea elevada
Imigran pode não ser adequado para si:
?Informa ao seu médico ou farmacêutico antes de usar Imigran.
Se padece doença renal ou hepática
?Informa ao seu médico ou farmacêutico antes de usar Imigran.
Se é alérgico aos antibióticos denominados sulfonamidas
Se for assim, você também pode ser alérgico a Imigran. Se você sabe que é alérgico a antibióticos, mas não tem certeza se se trata de uma sulfonamida:
?Informa ao seu médico ou farmacêutico antes de usarImigran.
Se está tomando antidepressivos denominados ISRSs(Inibidores Seletivos da Recaptura de Serotonina)ou IRSNs(Inibidores da Recaptura de Serotonina e Noradrenalina)
?Informa ao seu médico ou farmacêutico antes de usarImigran. Ver também abaixo, Outros medicamentos e Imigran.
Se usaImigranfrequentemente
Um uso excessivo de Imigran pode piorar as suas dores de cabeça.
?Informa ao seu médico se isto lhe acontece.O seu médico pode recomendar-lhe que deixe de usar este medicamento.
Se sente dor ou opressão no peito após usar Imigran
Estes efeitos podem intensificar-se, mas normalmente passam rapidamente. Se não passam rapidamente ou se tornam mais graves:
?Obtenha ajuda médica imediatamente.Ver a secção 4 deste prospecto que contém mais informações sobre estes possíveis efeitos adversos.
Outros medicamentos e Imigran
Informaao seu médico ou farmacêutico se está tomando, tomou recentemente ou possa ter que tomar qualquer outro medicamento. Isto inclui qualquer produto à base de plantas medicinais ou medicamentos que tenham sido comprados sem receita médica.
Alguns medicamentos não devem ser tomados com Imigran e outros podem causar efeitos adversos se forem tomados com Imigran. Informa ao seu médico se está tomando outros medicamentos, tais como:
- ergotaminausada também para tratar a enxaqueca,ou medicamentos semelhantes como metisergida (ver a secção 2 Não useImigran). Não use Imigran ao mesmo tempo que estes medicamentos. Deixe de tomar estes medicamentos pelo menos 24 horas antes de usar Imigran. Não tome nenhum outro medicamento que contenha ergotamina ou compostos semelhantes à ergotamina até pelo menos 6 horas após usar Imigran
- outros triptanos/agonistas do receptor 5-HT1(tais como naratriptano, rizatriptano, zolmitriptano), também usados para tratar a enxaqueca,(ver a secção 2 Não useImigran). Não use Imigran ao mesmo tempo que estes medicamentos. Deixe de tomar estes medicamentos pelo menos 24 horas antes de usar Imigran. Não tome outro triptano/agonista do receptor 5-HT1 pelo menos 24 horas após usar Imigran
- ISRSs(Inibidores Seletivos da Recaptura de Serotonina)ou IRSNs(Inibidores da Recaptura de Serotonina e Noradrenalina)usados para tratar a depressão. O uso de Imigran com estes medicamentos pode causar síndrome serotoninérgica (um conjunto de sintomas que podem incluir inquietude, confusão, suor, alucinações, aumento dos reflexos, espasmos musculares, arrepios, aumento dos batimentos cardíacos e agitação). Informa ao seu médico imediatamente se for afetado desta forma
- IMAOs(inibidores da monoamino oxidase)usados para tratar a depressão. Não use Imigran se tomou estes medicamentos nas últimas 2 semanas
- Erva de São João(Hypericum perforatum). É mais provável sofrer efeitos adversos se tomar remédios à base de plantas medicinais que contenham a Erva de São João enquanto está usando Imigran.
Fertilidade, gravidez e amamentação
Se está grávida ou em período de amamentação, acredita que possa estar grávida ou tem intenção de engravidar, consulte o seu médico antes de utilizar este medicamento. Há informações limitadas sobre a segurança de Imigran em mulheres grávidas, embora até agora não haja provas de que aumente o risco de defeitos no nascimento. O seu médico avaliará com você se deve ou não usar Imigran enquanto está grávida.
Não deve dar o peito ao seu bebê antes de que transcorram12 horas desde que tomou Imigran. Se extrair o leite materno durante este tempo, descarte o leite e não o dê ao seu bebê.
Condução e uso de máquinas
Este medicamento, assim como a enxaqueca, pode causar sonolência. Se nota estes efeitos, evite conduzir ou utilizar máquinas, pois pode ser perigoso.
Imigran contém cloreto de sódio
Este medicamento contém menos de 23 mg de sódio (1 mmol) por 0,5 ml; isto é, é essencialmente “exento de sódio”.
Pode produzir reações alérgicas graves porque contém goma de látex no envase.
3. Como usar Imigran
Siga exatamente as instruções de administração deste medicamento indicadas pelo seu médico. Em caso de dúvida, consulte novamente o seu médico ou farmacêutico.
Apenas use Imigran após o início da sua cefaleia migrañosa. Não use Imigran para prevenir o ataque de enxaqueca.
Lembre-se de usar o seu medicamento.
O seu médico indicar-lhe-á a duração do seu tratamento com Imigran. Não suspenda o tratamento antes.
Imigran deve ser usado assim que apareçam os sintomas de enxaqueca ou cefaleia em salvas; no entanto, pode ser usado em qualquer momento durante um ataque.
Não se recomenda a administração a crianças, adolescentes menores de 18 anos nem a pacientes de idade avançada.
A dose recomendada em adultos para os sintomas de enxaqueca ou de cefaleia em salvas é de uma única injeção (6 mg) subcutânea, justo abaixo da pele. O seu médico ensinar-lhe-á onde administrar a injeção, normalmente na coxa ou na nádega. Não coloque a injeção diretamente numa veia. Algumas pessoas começam a notar uma melhoria aos 15 minutos da administração de Imigran.
Se não se obtém melhoria após a administração de uma dose, não se deve usar uma segunda dose de sumatriptano para o mesmo ataque de enxaqueca ou cefaleia em salvas. No entanto, poderá tomar o seu medicamento habitual para a dor de cabeça, desde que este não contenha ergotamina ou dihidroergotamina.
Se se obtém uma melhoria dos sintomas após a primeira dose, mas os sintomas reaparecem, pode usar-se uma segunda dose de Imigran, uma vez transcorrida pelo menos uma hora desde a primeira dose.
Não use mais de 2 injeções em 24 horas e deixe transcorrer pelo menos uma hora entre cada dose.
As agulhas e as seringas podem ser perigosas e devem ser descartadas de forma segura e higiênica.

 O dispositivo consta de três partes: autoinjetor, petaca ou estuche e um cartucho que contém duas seringas, tal como se observa na Figura seguinte:
O dispositivo consta de três partes: autoinjetor, petaca ou estuche e um cartucho que contém duas seringas, tal como se observa na Figura seguinte:
O autoinjetor poderá ser utilizado apenas quando estiver carregado com uma seringa.
Conteúdo do estuche ou petaca
- Ao abrir a tampa do estuche ou petaca, poderá ver o autoinjetor e o cartucho com as seringas.
- Verifique que o cartucho está corretamente colocado, isto é, quando se vêem os botões azuis através dos orifícios do estuche ou petaca.
Para usar o autoinjetor

- Abrir o estuche ou petaca.
Retire o precinto de uma das duas seringas
que contém o cartucho.
- Abrir a tampa da seringa correspondente.
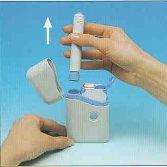
- Retirar o autoinjetor do estuche ou petaca.

- Introduzir com firmeza o autoinjetor no cartucho e girar o autoinjetor no sentido dos ponteiros do relógio até não poder girar mais.

- Puxar com firmeza o autoinjetor carregado para fora. É possível que lhe custe um pouco fazer isso.
Há um botão de segurança que evita uma injeção acidental até que você esteja preparado.
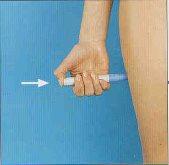
- Pressionar firmemente o autoinjetor carregado contra a pele, - preferentemente na face externa da coxa ou na parte superior da face externa do braço (músculo deltoides) -, de forma que a parte gris deslize justo até a parte azul. Para injetar, apertar o botão azul do autoinjetor e mantê-lo assim fixo durante cinco segundos pelo menos (ou contar até dez).
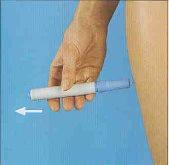
- Cuidadosamente retirar o autoinjetor.
Tenha cuidado, pois verá a agulha.

- Imediatamente devolver a seringa utilizada colocando-a no cartucho, empurrando o autoinjetor para baixo no interior do mesmo até onde chegar. Em seguida, girar o autoinjetor no sentido contrário ao dos
ponteiros do relógio até que saia do cartucho.

- Retirar o autoinjetor do cartucho; em seguida, fechar a tampa sobre a seringa utilizada.

- Devolver o autoinjetor ao seu lugar no estuche ou petaca empurrando-o para baixo. Ouviará um som de clique quando o autoinjetor estiver bem colocado.
Se usar mais Imigran do que deve:
Em caso de sobredose ou ingestão acidental, consulte imediatamente o seu médico ou farmacêutico ou ligue para o Serviço de Informação Toxicológica, telefone: 91 562 04 20, indicando o medicamento e a quantidade ingerida.
Se tiver alguma outra dúvida sobre o uso deste medicamento, pergunte ao seu médico ou farmacêutico.
4. Efeitos adversos possíveis
Tal como todos os medicamentos, o Imigran pode produzir efeitos adversos, embora nem todas as pessoas os sofram. Alguns destes sintomas podem ser causados pela própria enxaqueca.
Reações alérgicas: obtenha assistência médica rapidamente
Os seguintes efeitos adversos ocorreram em um número muito pequeno de pessoas e a sua frequência exata é desconhecida.
- Os sinais de alergia incluem erupção, habões (coceira e erupção); sibilâncias (assobios no peito); pálpebras, face ou lábios inchados, colapso completo.
Se notar algum desses sintomas pouco após utilizar o Imigran:
? Nunca mais o utilize.Entre em contato rapidamente com o seu médico.
Efeitos adversos muito frequentes
(afetam mais de 1 em cada 10pessoas)
- Dor no local da injeção.
- Coceira/queimadura, vermelhidão, inchaço, hematoma e sangramento no local da injeção.
Efeitos adversos frequentes
(afetam até 1 em cada 10pessoas)
- Dor, pesadez, pressão, opressão ou dor no peito, garganta ou outras partes do corpo, ou sensações incomuns, incluindo formigamento, entorpecimento e calor ou frio. Estes efeitos podem ser intensos, mas geralmente desaparecem rapidamente.
Se estes efeitos continuarem ou piorarem(especialmente dor no peito):
?Obtenha assistência médica urgentemente. Em um número reduzido de pessoas, estes sintomas podem ser causados por ataques cardíacos.
Outros efeitos adversos frequentes incluem:
- Náuseas ou vômitos, embora isso possa ser devido à própria enxaqueca.
- Cansaço ou sonolência.
- Tonturas, sensação de fraqueza ou sofocos.
- Aumento temporário da pressão sanguínea.
- Dificuldade para respirar.
- Dor muscular.
Efeitos adversos muito raros
(afetam até 1 em cada 10.000pessoas)
- Alterações na função do fígado. Avise o seu médico ou enfermeira de que está tomando Imigran, se for realizar uma análise de sangue para verificar o funcionamento do fígado.
Alguns pacientes podem experimentar algum dos seguintes efeitos adversos, mas a frequência com que ocorrem é desconhecida
- Convulsões/ataques, tremores, espasmo muscular, rigidez de pescoço.
- Alterações visuais, como piscar, visão reduzida, visão dupla, perda de visão e, em alguns casos, até defeitos permanentes (embora estes possam ser devidos ao ataque de enxaqueca em si).
- Problemas cardíacos, nos quais o coração pode bater mais rápido, mais lento ou com alterações no ritmo, dor no peito (angina) ou ataque cardíaco.
- Palidez, pele azulada e/ou dor nos dedos das mãos, dedos dos pés, orelhas, nariz ou mandíbula em resposta ao frio ou ao estresse (fenômeno de Raynaud).
- Dor na parte inferior esquerda do abdômen e diarreia sanguinolenta (colite isquêmica).
- Diarreia.
- Se teve uma lesão recente ou se tem inflamação (como reumatismo ou inflamação do cólon), pode experimentar dor ou piora da dor no local da lesão ou inflamação.
- Dor nas articulações.
- Ansiedade.
- Dificuldade para engolir.
- Sudorese excessiva.
Se foi realizado um exame de sangue para verificar como funciona o seu fígado e tomou Imigran, comunique ao seu médico, pois pode afetar os resultados.
Comunicação de efeitos adversos:
Se experimentar qualquer tipo de efeito adverso, consulte o seu médico ou farmacêutico, mesmo que se trate de possíveis efeitos adversos que não aparecem neste prospecto. Também pode comunicá-los diretamente através do Sistema Espanhol de Farmacovigilância de Medicamentos de Uso Humano: www.notificaRAM.es. Mediante a comunicação de efeitos adversos, você pode contribuir para fornecer mais informações sobre a segurança deste medicamento.
5. Conservação do Imigran
Mantenha este medicamento fora da vista e do alcance das crianças.
Não conserve a uma temperatura superior a 30ºC. Conserve no embalagem original para protegê-lo da luz.
Não utilize este medicamento após a data de validade que aparece no envase após CAD. A data de validade é o último dia do mês que se indica.
Os medicamentos não devem ser jogados nos deságues ou na lixeira. Deposite os envases e os medicamentos que não precisa no Ponto SIGRE da farmácia. Em caso de dúvida, pergunte ao seu farmacêutico como se livrar dos envases e dos medicamentos que não precisa. Dessa forma, ajudará a proteger o meio ambiente.
6. Conteúdo do envase e informações adicionais
Composição do Imigran:
- O princípio ativo é sumatriptano. Cada seringa pré-carregada contém 6 miligramas de sumatriptano (como sumatriptano succinato).
- Os demais componentes são cloreto de sódio e água para preparações injetáveis.
Aspecto do produto e conteúdo do envase
O Imigran é apresentado na forma de seringa pré-carregada com uma solução que contém 6 mg de sumatriptano. Cada envase contém um estojo ou petaca com 2 seringas pré-carregadas e um dispositivo auto-injetor.
O protetor da agulha da seringa pré-carregada contém borracha de látex natural seca que pode produzir reações alérgicas graves em pessoas sensíveis ao látex.
Título da autorização de comercialização e responsável pela fabricação
Título da autorização de comercialização:
GlaxoSmithKline S.A.
P.T.M. C/ Severo Ochoa, 2
28760 Tres Cantos (Madrid)
Tel: +34 900 202 700
Responsável pela fabricação:
GlaxoSmithKline Manufacturing S.p.A.Strada Provinciale Asolana, 90 43056 San Polo di Torrile Parma Itália
Data da última revisão deste prospecto:setembro 2020.
A informação detalhada e atualizada deste medicamento está disponível na página Web da Agência Espanhola de Medicamentos e Produtos Sanitários (AEMPS) http://www.aemps.gob.es/

Quanto custa o IMIGRAN 6 mg solução injetável em Espanha em 2025?
O preço médio do IMIGRAN 6 mg solução injetável em dezembro de 2025 é de cerca de 26.21 EUR. Os valores podem variar consoante a região, a farmácia e a necessidade de receita. Confirme sempre com uma farmácia local ou fonte online para obter informações atualizadas.
- País de registo
- Preço médio em farmácia26.21 EUR
- Substância ativa
- Requer receita médicaSim
- Fabricante
- Esta informação é apenas para referência e não constitui aconselhamento médico. Consulte sempre um médico antes de tomar qualquer medicamento. A Oladoctor não se responsabiliza por decisões médicas baseadas neste conteúdo.
- Alternativas a IMIGRAN 6 mg solução injetávelForma farmacêutica: PRODUTO PARA USO NASAL, 10 mgSubstância ativa: sumatriptanFabricante: Glaxosmithkline S.A.Requer receita médicaForma farmacêutica: PRODUTO PARA USO NASAL, 20 mg de sumatriptanoSubstância ativa: sumatriptanFabricante: Glaxosmithkline S.A.Requer receita médicaForma farmacêutica: COMPRIMIDO, 50 mgSubstância ativa: sumatriptanFabricante: Glaxosmithkline S.A.Requer receita médica
Alternativas a IMIGRAN 6 mg solução injetável noutros países
As melhores alternativas com o mesmo princípio ativo e efeito terapêutico.
Alternativa a IMIGRAN 6 mg solução injetável em Polónia
Alternativa a IMIGRAN 6 mg solução injetável em Ukraine
Médicos online para IMIGRAN 6 mg solução injetável
Avaliação de posologia, efeitos secundários, interações, contraindicações e renovação da receita de IMIGRAN 6 mg solução injetável – sujeita a avaliação médica e regras locais.














