HIZENTRA 200 mg/mL Solução Injetável Subcutânea

Pergunte a um médico sobre a prescrição de HIZENTRA 200 mg/mL Solução Injetável Subcutânea

Como usar HIZENTRA 200 mg/mL Solução Injetável Subcutânea
Introdução
Prospecto: informação para o utilizador
Hizentra 200 mg/ml solução injetável subcutânea
imunoglobulina humana normal (IgSC = Inmunoglobulina Subcutânea)
Leia todo o prospecto atentamente antes de começar a usar este medicamento, porque contém informações importantes para si.
- Conserva este prospecto, porque pode ter que voltar a lê-lo.
- Se tiver alguma dúvida, consulte o seu médico, farmacêutico ou enfermeiro.
- Este medicamento foi prescrito apenas para si e não deve dá-lo a outras pessoas, mesmo que apresentem os mesmos sintomas de doença que si, porque pode prejudicá-las.
- Se experimentar efeitos adversos, consulte o seu médico, farmacêutico ou enfermeiro, mesmo que se trate de efeitos adversos que não aparecem neste prospecto. Ver seção 4.
Conteúdo do prospecto
- O que é Hizentra e para que é utilizado
- O que necessita saber antes de começar a usar Hizentra
- Como usar Hizentra
- Posíveis efeitos adversos
- Conservação de Hizentra
- Conteúdo do envase e informações adicionais
1. O que é Hizentra e para que é utilizado
O que é Hizentra
Hizentra pertence à classe de medicamentos chamados imunoglobulinas humanas normais. As imunoglobulinas também são conhecidas como anticorpos e são proteínas do sangue que ajudam o organismo a lutar contra as infecções.
Como actua Hizentra
Hizentra contém imunoglobulinas que foram obtidas do sangue de pessoas saudáveis. As imunoglobulinas são produzidas pelo sistema imunológico do corpo. Ajudam o corpo a combater infecções causadas por bactérias e vírus ou a manter o equilíbrio no sistema imunológico (conhecido como imunomodulação). O medicamento funciona exatamente igual às imunoglobulinas presentes de forma natural no sangue.
Para que é utilizado Hizentra
Terapia substitutiva
Hizentra é utilizado para elevar os níveis anormalmente baixos de imunoglobulina no sangue até os níveis normais (terapia substitutiva). O medicamento é usado em adultos e crianças (0-18 anos) nas situações seguintes:
- Tratamento de pacientes que nascem com uma menor capacidade ou com incapacidade para produzir imunoglobulinas (imunodeficiências primárias). Isso inclui condições do tipo:
- Níveis baixos de imunoglobulina (hipogamaglobulinemia) ou ausência de imunoglobulinas no sangue (agamaglobulinemia)
- Combinação de níveis baixos de imunoglobulinas, infecções frequentes e incapacidade de produzir os níveis adequados de anticorpos após uma vacinação (imunodeficiência comum variável)
- Combinação de níveis baixos ou ausência de imunoglobulinas, e ausência ou não funcionalidade das células imunes (imunodeficiência combinada grave)
- Falta de certas subclases de imunoglobulina G que provocam infecções recorrentes.
- Tratamento de pacientes com níveis de imunoglobulina baixos ou disfuncionais em condições adquiridas (imunodeficiência secundária) que experimentam infecções graves ou recorrentes devido a um sistema imunológico debilitado como resultado de outras condições ou tratamentos.
Terapia imunomoduladora em pacientes com PDIC
Hizentra também é utilizado em pacientes com polineuropatia desmielinizante inflamatória crónica (PDIC), um tipo de doença autoimune. PDIC se caracteriza pela inflamação crônica dos nervos periféricos que causam fraqueza muscular e/ou formigamento principalmente em pernas e braços. Acredita-se que o ataque das defesas do corpo acentua essa inflamação e as imunoglobulinas de Hizentra ajudam a proteger os nervos do ataque (terapia imunomoduladora).
2. O que necessita saber antes de começar a usar Hizentra
NÃOperfuse Hizentra:
- Se é alérgico às imunoglobulinas humanas, ao polissorbato 80 ou à L-prolina.
- Informa ao seu médico ou profissional de saúde antes do tratamento se sofreu alguma intolerância a algum desses componentes anteriormente.
- Se sofre de hiperprolinemia (um distúrbio genético causado pela presença no sangue de altos níveis do aminoácido prolina).
Em vasos sanguíneos.
Advertências e precauções
- Consulte o seu médico, farmacêutico ou enfermeiro antes de usar Hizentra.
Você pode ser alérgico (hipersensível) às imunoglobulinas sem saber. No entanto, as reações alérgicas verdadeiras são raras. Podem ocorrer mesmo que tenha recebido anteriormente imunoglobulinas humanas e as tenha tolerado bem. Isso pode ocorrer especialmente se não tiver suficiente quantidade da imunoglobulina tipo A (IgA) no sangue (deficiência de IgA).
- Informa ao seu médico ou profissional de saúde se padece de deficiência de imunoglobulina de tipo A (IgA) antes de iniciar o tratamento. Hizentra contém quantidades residuais de IgA que podem provocar reações alérgicas.
Nestes casos raros, podem ocorrer reações alérgicas, como uma diminuição súbita da tensão arterial ou um choque(ver também seção 4. "Posíveis efeitos adversos").
- Se nota sintomas deste tipo durante a perfusão de Hizentra, detenha a perfusão e contacte com o seu médico ou vá ao hospital mais próximo de forma imediata.
- Informa ao seu médico se tem antecedentes de afecção cardíaca ou vascular, ou se sofreu a formação de coágulos sanguíneos, tem o sangue muito espesso ou esteve imobilizado durante algum tempo. Estes fatores podem aumentar o seu risco de sofrer um coágulo sanguíneo após tomar Hizentra. Assim como, indique ao seu médico quais medicamentos está tomando, porque alguns contêm estrógenos (uma hormona que se encontra, por exemplo, nas píldoras anticonceptivas) que podem aumentar o seu risco de desenvolver um coágulo sanguíneo. Ponha-se imediatamente em contacto com o seu médico se experimenta sinais e sintomas como dificuldade para respirar, dor no peito, dor e inflamação de uma extremidade, fraqueza ou adormecimento de um lado do corpo após ter tomado Hizentra.
- Ponha-se em contacto com o seu médico se experimenta os seguintes sinais e sintomas: dor de cabeça aguda, rigidez de pescoço, sonolência, febre, fotofobia, náuseas e vómitos após ter tomado Hizentra. O seu médico decidirá se é necessário realizar mais testes e se deve continuar a tomar o tratamento com Hizentra.
O seu profissional de saúde evitará possíveis complicações verificando que:
- Você não é sensível à imunoglobulina humana normal.
O produto deve ser injetado a uma velocidade lenta inicialmente. Deve respeitar-se estritamente a velocidade de injeção recomendada no apartado 3 “Como usar Hizentra”.
- É vigiada minuciosamente a presença de qualquer sintoma durante todo o período de
perfusão, especialmente se:
- é a primeira vez que recebe imunoglobulina humana normal
- mudou de tratamento com outro medicamento
- passou um período prolongado (mais de oito semanas) desde a última perfusão que recebeu.
Nestes casos, deve ser observado durante a primeira perfusão e durante uma hora após a mesma. Se os pontos acima mencionados não podem aplicar-se a você, recomenda-se a sua observação durante pelo menos 20 minutos após a administração.
Interacção de Hizentra com outros medicamentos
- Informa ao seu médico ou farmacêutico se está utilizando, utilizou recentemente ou poderia ter que utilizar qualquer outro medicamento.
- Não deve misturar Hizentra com outros medicamentos.
- Antes de se vacinar, informa ao seu médico acerca do seu tratamento com Hizentra.
Hizentra pode alterar o efeito de algumas vacinas com vírus vivos como a vacina contra o sarampo, rubéola, parotidite e varicela. Por isso, após a administração destes medicamentos, deve esperar pelo menos 3 meses antes de receber uma vacina viva atenuada. No caso da vacina contra o sarampo, esta alteração pode persistir até 1 ano.
Gravidez, lactação e fertilidade
- Se está grávida ou em período de lactação, ou acredita que possa estar grávida ou tem intenção de ficar grávida, informa ao seu médico ou farmacêutico. O seu médico decidirá se pode receber Hizentra durante a gravidez ou enquanto estiver a dar o peito.
Não foram desenvolvidos estudos com Hizentra em mulheres grávidas. No entanto, foram utilizados medicamentos que contêm imunoglobulina em mulheres grávidas ou em período de lactação há anos e não foram observados efeitos prejudiciais no curso da gravidez ou no bebê.
Se está a dar o peito e recebe Hizentra, as imunoglobulinas do medicamento também podem estar presentes no leite materno. Por isso, o seu bebê pode estar protegido contra algumas infecções.
Condução e uso de máquinas
Durante o tratamento com Hizentra, os pacientes podem experimentar efeitos, como tonturas ou náuseas, que poderiam afetar a capacidade para conduzir e utilizar máquinas. Se isso acontecer, não deve conduzir nem usar máquinas até que esses efeitos tenham desaparecido.
Hizentra contém prolina
Não deve tomar este medicamento se sofre de hiperprolinemia (ver também o apartado 2 “O que necessita saber antes de começar a usar Hizentra”). Informa ao seu médico antes do tratamento.
Outra informação importante sobre Hizentra
Análise de sangue
Após receber Hizentra, os resultados de alguns exames de sangue (testes serológicos) podem estar alterados durante algum tempo.
- Antes de realizar qualquer exame de sangue, informa ao seu médico acerca do seu tratamento com Hizentra.
Informação acerca dos componentes de Hizentra
Hizentra é elaborado a partir de plasma de sangue humano (esta é a parte líquida do sangue). Quando os medicamentos são fabricados a partir do sangue ou do plasma humano, são postas em prática certas medidas para evitar a transmissão de infecções aos pacientes. Estas medidas incluem:
- uma seleção meticulosa dos doadores de sangue ou plasma para garantir a exclusão dos possíveis portadores de infecções, e
- o exame de cada doação e das misturas de plasmas para comprovar a ausência de sinais de vírus ou infecções.
Os fabricantes destes medicamentos também incluem passos no processamento do sangue ou do plasma que podem desativar ou eliminar os vírus. Apesar destas medidas, ao administrar medicamentos preparados a partir de sangue ou plasma humano, não se pode excluir completamente a possibilidade de transmitir uma infecção. Isso também é verdadeiro no caso de qualquer vírus desconhecido ou que apareça, ou de qualquer outro tipo de infecção.
As medidas adoptadas são consideradas eficazes para os vírus com envoltura, como o vírus da imunodeficiência humana (VIH, o vírus da SIDA), o vírus da hepatite B e o vírus da hepatite C (inflamação do fígado), e para os vírus sem envoltura, como o vírus da hepatite A e o parvovirus B19.
- Recomenda-se encarecidamente que, cada vez que você receber uma dose de Hizentra, tome nota do nome e do número de lote do produto, com o fim de manter um registo dos lotes usados (ver a seção 3, “Como usar Hizentra”).
Hizentra contém sódio
Este medicamento contém menos de 23 mg de sódio (1 mmol) por frasco/seringa; isto é, é essencialmente “isento de sódio”.
3. Como usar Hizentra
Siga exatamente as instruções de administração deste medicamento indicadas pelo seu médico. Consulte o seu médico se tiver dúvidas.
Dose
O seu médico decidirá que quantidade de Hizentra você receberá, com base no seu peso e resposta ao tratamento.
A dose ou o intervalo de administração não devem ser alterados sem consultar o seu médico.
Se acredita que deve receber Hizentra com mais ou menos frequência, por favor, consulte o seu médico. Se acredita que esqueceu uma dose, fale com o seu médico o mais rápido possível.
Terapia substitutiva
O seu médico determinará se você precisa de uma dose de carga (para adultos e crianças) de pelo menos 1 a 2,5 ml/kg de peso corporal dividida em vários dias. Depois, serão administradas doses de manutenção a intervalos repetidos, de uma vez ao dia a uma vez a cada duas semanas, para alcançar uma dose mensal acumulada de entre 2 e 4 ml/kg de peso corporal. O seu profissional de saúde pode ajustar a dose com base na sua resposta ao tratamento.
Terapia imunomoduladora
O seu médico iniciará a terapia com Hizentra uma semana após a sua última perfusão intravenosa de imunoglobulina administrada sob a pele (por via subcutânea) com uma dose semanal de 1,0 a 2,0 ml/kg de peso corporal. O seu médico determinará a sua dose semanal de Hizentra. As doses semanais de manutenção podem ser divididas em doses menores e administradas com a frequência necessária durante a semana. Para esquemas a cada duas semanas, o seu médico duplicará a dose semanal de Hizentra. O seu profissional de saúde pode ajustar a dose com base na sua resposta ao tratamento.
Forma e via de administração
No caso do tratamento em casa, este deve ser iniciado por um profissional de saúde com experiência no tratamento de imunodeficiência/PDIC com IgSC e na instrução de pacientes para o tratamento em casa.
Ele o instruirá e treinará em:
- técnicas de perfusão asséptica,
- manutenção de um diário de tratamento, e
- medidas a tomar em caso de efeitos adversos graves.
Ponto(s) de perfusão
- Administre Hizentra apenas por via subcutânea.
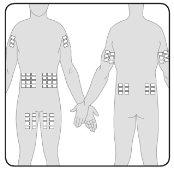
- Pode perfundir Hizentra em pontos como o abdômen, a coxa, o braço e a parte lateral da cintura. Em caso de doses altas (> 50 ml), administre em múltiplos pontos.
- Pode usar um número ilimitado de pontos de injeção de forma simultânea. Os pontos onde se aplicam as injeções devem estar separados por pelo menos 5 cm.
- Se usar a técnica de perfusão assistida por dispositivo (por exemplo, perfusão assistida por bomba), pode usar de forma simultânea mais de um dispositivo de perfusão.
- Se usar a técnica de perfusão por empurrão manual com uma seringa, pode usar apenas um local de perfusão por seringa. Se precisar administrar uma seringa adicional de Hizentra, deve usar uma nova agulha de injeção estéril e mudar o local de perfusão.
- O volume de produto perfundido em um local determinado pode variar.
Velocidade(s) de perfusão
O seu médico determinará a técnica de perfusão adequada e a velocidade de perfusão para você, considerando a sua dose individual, frequência de dosagem e tolerabilidade do produto.
Perfusão assistida por dispositivo:
A velocidade de perfusão inicial recomendada é de até 20 ml/hora por ponto. Se for bem tolerada, a velocidade de perfusão pode ser aumentada gradualmente para 35 ml/hora por ponto para as perfusões posteriores. A partir daí, a velocidade de perfusão pode ser aumentada de acordo com a sua tolerabilidade.
Perfusão por empurrão manual:
A velocidade de perfusão inicial recomendada é de até 0,5 ml/min por ponto (30 ml/hora por ponto). Se for bem tolerada, a velocidade de perfusão pode ser aumentada até 2,0 ml/min por ponto (120 ml/hora por ponto) para perfusões posteriores. A partir daí, a velocidade de perfusão pode ser aumentada ainda mais de acordo com a sua tolerabilidade.
Instruções de uso
Siga os seguintes passos e use uma técnica asséptica para administrar Hizentra. | ||
1 | Limpe a superfície Limpe a fundo a mesa ou outra superfície plana usando um pano antiséptico. | |
2 | Monte os acessórios Coloque Hizentra e o resto dos acessórios e equipamentos necessários para a perfusão sobre uma superfície limpa e plana. | |
3 | Lave e seque bem as mãos | |
4 | Verifique os frascos Inspeccione visualmente em Hizentra a presença de partículas na solução ou a decoloração da solução, bem como a data de validade antes de administrar Hizentra. Não utilize soluções turvas ou que contenham partículas. Não use soluções que tenham sido congeladas. Administre a solução quando estiver à temperatura ambiente ou à temperatura corporal. Uma vez aberto o frasco, use a solução imediatamente. | |
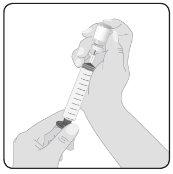
5 | Preparação de Hizentra para perfusão Limpe o batoque do frasco– Retire a cápsula protetora do frasco e deixe à vista a porção central do batoque de borracha. Limpe o batoque com um pano com álcool ou com um preparado antiséptico e deixe que seque. Transfira Hizentra para a seringa para perfusão– Acople o dispositivo de transferência ou a agulha a uma seringa estéril, usando uma técnica asséptica. Se utilizar um dispositivo de transferência (punção ventilada), siga as instruções indicadas pelo fabricante do dispositivo. Se utilizar uma agulha, empurre para trás o êmbolo para tomar ar na seringa em quantidade semelhante à quantidade de Hizentra que será retirada. Em seguida, insira a agulha no centro do batoque do frasco e, evitando a formação de espuma, injete o ar na câmara de ar do frasco (não no líquido). Finalmente, retire o volume desejado de Hizentra. Em caso de que utilize vários frascos para alcançar a dose desejada, repita este passo. |
|
6 | Prepare o conector Acople o conector de administração ou o equipamento de agulhas à seringa. Cebe o conector para eliminar o ar restante. | |
7 | Prepare o(s) ponto(s) de perfusão Selecione o(s) ponto(s) de perfusão –O número e a localização dos pontos de perfusão dependem do volume total da dose. Cada ponto de perfusão deve estar separado por pelo menos 5 cm. Pode utilizar um número ilimitado de pontos de perfusão de forma simultânea. |
|
Limpe o(s) ponto(s) de perfusãoutilizando uma preparação cutânea antiséptica. Deixe que cada ponto seque antes de proceder. | ||
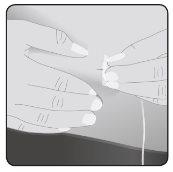
8 | Insira a agulha Tome a pele entre 2 dedos e insira a agulha no tecido subcutâneo. |
|
Asegure a agulha à pele– Se necessário, use uma gaze e esparadrapo ou curativo transparente para asegurar a agulha no lugar. | ||
9 | Perfunda Hizentra Inicie a perfusão. Se utilizar uma bomba de perfusão, siga as instruções do fabricante. | |
10 | Registre a perfusão Registre os seguintes dados no seu diário de tratamento:
| |
11 | Limpeza Descarte o produto não utilizado e todos os acessórios de administração utilizados após a administração de acordo com os requisitos locais. |
Se tiver alguma dúvida sobre o uso deste produto, consulte o seu médico, farmacêutico ou enfermeiro.
Se usar mais Hizentra do que deve
Informar ao seu médico o mais rápido possível se acredita que recebeu demasiado Hizentra.
Se esqueceu de usar Hizentra
Informar ao seu médico o mais rápido possível se acredita que esqueceu uma dose.
4. Possíveis efeitos adversos
Assim como todos os medicamentos, este medicamento pode produzir efeitos adversos, embora nem todas as pessoas os sofram.
- Em casos isolados, pode ser alérgico (hipersensível) às imunoglobulinas e podem ocorrer reações alérgicas como um descenso repentino da tensão arterial ou choque (p. ex., pode se sentir atordoado ou mareado, sentir mareio ao levantar, ter as mãos e os pés frios, uma sensação anormal de batimento cardíaco ou dor torácica ou ter visão borrada).
- Em casos isolados, pode experimentar dor e/ou inchaço de um braço ou perna com calor na zona afetada, decoloração de um braço ou perna, falta de ar inexplicável, dor torácica ou mal-estar que piora com a respiração profunda, pulso rápido inexplicável, entorpecimento ou fraqueza em um lado do corpo, confusão repentina ou dificuldade para falar ou entender, que poderiam ser sinais de um coágulo de sangue.
- Em casos isolados, pode ter um forte dor de cabeça com náuseas, vômitos, rigidez no pescoço, febre e sensibilidade à luz, que poderiam ser sinais de SMA (síndrome de meningite asséptica), que é uma inflamação temporária não infecciosa reversível das membranas que rodeiam o cérebro e a medula espinhal.
- Se notar tais sinais durante a perfusão de Hizentra, interrompa a perfusão e vá ao hospital mais próximo de forma imediata.
Ver também a seção 2 deste prospecto sobre o risco de reações alérgicas, coágulos de sangue e SMA.
Os efeitos adversos observados nos ensaios clínicos controlados são apresentados em ordem decrescente de frequência. Os efeitos adversos observados pós-comercialização são de frequência desconhecida:
Os seguintes efeitos adversos são muito frequentes(afetam mais de 1 de cada 10 pacientes):
- Dor de cabeça
- Erupção
- Reações na zona de perfusão
Os seguintes efeitos adversos são frequentes(afetam entre 1 e 10 pacientes de cada 100):
- Tontura
- Enxaqueca
- Pressão arterial aumentada (hipertensão)
- Diarréia
- Dor abdominal
- Mal-estar geral (náuseas)
- Vômitos
- Coceira (prurido)
- Urticárias
- Dor relacionada aos músculos e ossos (dor musculoesquelética)
- Febre
- Dor articular (artralgia)
- Cansaço (fadiga), que inclui indisposição (mal-estar geral)
- Dor no peito
- Sintomas semelhantes aos da gripe
- Dor
Os seguintes efeitos adversos são pouco frequentes(afetam entre 1 e 10 pacientes de cada 1.000):
- Hipersensibilidade
- Movimentos de agitação involuntários em uma ou mais partes do corpo (temores, incluindo hiperatividade psicomotora)
- Batimento cardíaco rápido (taquicardia)
- Verdura
- Espasmos musculares
- Fraqueza muscular
- Calafrios, que inclui temperatura corporal baixa
- Resultados anormais de análise de sangue que podem indicar alterações das funções hepática e renal
Em casos isolados, pode ocorrer uma úlcera no local de perfusão ou sensação de ardor.
- Pode reduzir possíveis efeitos adversos se perfundir lentamente Hizentra.
Estes efeitos adversos podem ocorrer embora você tenha recebido anteriormente imunoglobulinas humanas e as tenha tolerado bem.
Consulte também a seção 2 "O que precisa saber antes de começar a usar Hizentra", onde encontrará informações adicionais sobre os fatores que podem aumentar o risco de sofrer efeitos adversos.
Comunicação de efeitos adversos
Se experimentar qualquer tipo de efeito adverso, consulte o seu médico ou farmacêutico, mesmo que se trate de efeitos adversos que não aparecem neste prospecto. Também pode comunicá-los diretamente através do Sistema Espanhol de Farmacovigilância de Medicamentos de Uso Humano: www.notificaRAM.es.
Ao comunicar efeitos adversos, você pode contribuir para fornecer mais informações sobre a segurança deste medicamento.
5. Conservação de Hizentra
- Mantenha este medicamento fora da vista e do alcance das crianças.
- Não utilize este medicamento após a data de validade que aparece no envase e na etiqueta do frasco após EXP.
- Deve utilizar/perfundir este medicamento o mais rápido possível após abrir o frasco. Não utilize Hizentra se o frasco estiver aberto ou defeituoso.
- Não conserve a temperatura superior a 25 °C.
- Não congele.
- Conservar o frasco no embalagem exterior para protegê-lo da luz.
- Não jogue os medicamentos pelos deságues ou na lixeira. Pergunte ao seu farmacêutico onde jogar os medicamentos que já não utiliza. Dessa forma, ajudará a proteger o meio ambiente.
6. Conteúdo do envase e informações adicionais
Composição de Hizentra
- O princípio ativoé a imunoglobulina humana normal. Um ml contém 200 mg de imunoglobulina humana normal, da qual pelo menos 98% é imunoglobulina do tipo G (IgG).
A porcentagem aproximada das subclasses de IgG é a seguinte:
IgG1 .................................. 69%
IgG2 .................................. 26%
IgG3 .................................. 3%
IgG4 .................................. 2%
Este medicamento contém traços de IgA (não mais de 50 microgramas/ml).
- Os demais componentes(excipientes) são L-prolina, polissorbato 80 e água para preparações injetáveis.
Aspecto de Hizentra e conteúdo do envase
Hizentra é uma solução injetável subcutânea (200 mg/ml). A cor pode variar de amarelo pálido a marrom claro.
Hizentra está disponível em frascos de 5, 10, 20 ou 50 ml.
Hizentra também está disponível em seringas pré-carregadas de 5, 10, 20 e 50 ml.
Tamanhos de envase
Pacotes de 1, 10 ou 20 frascos.
Hizentra também está disponível em envases de 1 (para 5, 10, 20, 50 ml) ou 10 (para 5, 10, 20 ml) ou 20 (para 10, 20 ml) seringas pré-carregadas.
Por favor, note que este envase não contém toalhetes com álcool, agulhas ou outros acessórios ou equipamentos.
Pode ser que apenas alguns tamanhos de envase estejam comercializados.
Titular da autorização de comercialização e responsável pela fabricação
CSL Behring GmbH
Emil-von-Behring-Strasse 76
D-35041 Marburg
Alemanha
Pode solicitar mais informações sobre este medicamento dirigindo-se ao representante local do titular da autorização de comercialização:
Bélgica CSL Behring NV Tel: +32 15 28 89 20 | Luxemburgo CSL Behring NV Tel: +32 15 28 89 20 |
| Hungria CSL Behring Kft. Tel: +36 1 213 4290 |
República Tcheca CSL Behring s.r.o. Tel: + 420 702 137 233 | Malta AM Mangion Ltd. Tel: +356 2397 6333 |
Dinamarca CSL Behring AB Tel: +46 8 544 966 70 | Países Baixos CSL Behring BV Tel: + 31 85 111 96 00 |
Alemanha CSL Behring GmbH Tel: +49 69 30584437 | Noruega CSL Behring AB Tel: +46 8 544 966 70 |
Estônia CentralPharma Communications OÜ Tel: +3726015540 | Áustria CSL Behring GmbH Tel: +43 1 80101 2463 |
Grécia CSL Behring ΕΠΕ Tel: +30 210 7255 660 | Polônia CSL Behring Sp. z o.o. Tel: +48 22 213 22 65 |
Espanha CSL Behring S.A. Tel: +34 933 67 1870 | Portugal CSL Behring Lda Tel: +351 21 782 62 30 |
França CSL Behring SA Tel: + 33 1 53 58 54 00 | Romênia Prisum Healthcare S.R.L. Tel: +40 21 322 01 71 |
Croácia Marti Farm d.o.o. Tel: +385 1 5588297 | Eslovênia EMMES BIOPHARMA GLOBAL s.r.o. - podružnica v Sloveniji Tel: +386 41 42 0002 |
Irlanda CSL Behring GmbH Tel: +49 69 305 17254 | Eslováquia CSL Behring Slovakia s.r.o. Tel: +421 911 653 862 |
Islândia CSL Behring AB Tel: +46 8 544 966 70 | Finlândia CSL Behring AB Tel: +46 8 544 966 70 |
Itália CSL Behring S.p.A. Tel: +39 02 34964 200 | Suécia CSL Behring AB Tel: +46 8 544 966 70 |
Chipre CSL Behring ΕΠΕ Tel: +30 210 7255 660 | Reino Unido (Irlanda do Norte) CSL Behring GmbH Tel: +49 69 30517254 |
Letônia CentralPharma Communications SIA Tel: +371 6 7450497 | |
Lituânia CentralPharma Communications UAB Tel: +370 5 243 0444 |
Data da última revisão deste prospecto:
A informação detalhada deste medicamento está disponível na página web da Agência Europeia de Medicamentos: http://www.ema.europa.eu/.
----------------------------------------------------------------------------------------------------------------
- País de registo
- Substância ativa
- Requer receita médicaSim
- Fabricante
- Esta informação é apenas para referência e não constitui aconselhamento médico. Consulte sempre um médico antes de tomar qualquer medicamento. A Oladoctor não se responsabiliza por decisões médicas baseadas neste conteúdo.
- Alternativas a HIZENTRA 200 mg/mL Solução Injetável SubcutâneaForma farmacêutica: INJETÁVEL, 165 mg/mlSubstância ativa: immunoglobulins, normal human, for extravascular adm.Fabricante: Octapharma S.A.Requer receita médicaForma farmacêutica: INJETÁVEL, 200 mg/mlSubstância ativa: immunoglobulins, normal human, for extravascular adm.Fabricante: Baxalta Innovations GmbhRequer receita médicaForma farmacêutica: INJETÁVEL, 200 mg/mlSubstância ativa: immunoglobulins, normal human, for extravascular adm.Fabricante: Csl Behring GmbhRequer receita médica
Alternativas a HIZENTRA 200 mg/mL Solução Injetável Subcutânea noutros países
As melhores alternativas com o mesmo princípio ativo e efeito terapêutico.
Alternativa a HIZENTRA 200 mg/mL Solução Injetável Subcutânea em Polska
Alternativa a HIZENTRA 200 mg/mL Solução Injetável Subcutânea em Ukraina
Médicos online para HIZENTRA 200 mg/mL Solução Injetável Subcutânea
Avaliação de posologia, efeitos secundários, interações, contraindicações e renovação da receita de HIZENTRA 200 mg/mL Solução Injetável Subcutânea – sujeita a avaliação médica e regras locais.