FULPHILA 6 mg SOLUÇÃO INJETÁVEL EM SERINGA PREENCHIDA

Como usar FULPHILA 6 mg SOLUÇÃO INJETÁVEL EM SERINGA PREENCHIDA
Introdução
Prospecto: informação para o utilizador
Fulphila 6 mg solução injetável em seringa pré-carregada
Pegfilgrastim
Leia todo o prospecto detenidamente antes de começar a usar este medicamento porque contém informações importantes para si.
- Conserva este prospecto, pois pode ter que voltar a lê-lo.
- Se tiver alguma dúvida, consulte o seu médico, farmacêutico ou enfermeiro.
- Este medicamento foi-lhe prescrito apenas para si e não deve dá-lo a outras pessoas, mesmo que tenham os mesmos sintomas que si, pois pode prejudicá-las.
- Se experimentar efeitos adversos, consulte o seu médico, farmacêutico ou enfermeiro, mesmo que se trate de efeitos adversos que não aparecem neste prospecto. Ver secção 4.
Conteúdo do prospecto
- O que é Fulphila e para que é utilizado
- O que necessita de saber antes de começar a usar Fulphila
- Como usar Fulphila
- Possíveis efeitos adversos
- Conservação de Fulphila
- Conteúdo do envase e informação adicional
1. O que é Fulphila e para que é utilizado
Fulphila contém o princípio ativo pegfilgrastim. Pegfilgrastim é uma proteína produzida por biotecnologia na bactéria E. coli. Pegfilgrastim pertence a um grupo de proteínas chamadas citocinas, e é muito semelhante a uma proteína natural (fator estimulador de colônias de granulócitos) que o nosso organismo produz.
Fulphila é utilizado para reduzir a duração da neutropenia (contagem baixa de glóbulos brancos) e a incidência da neutropenia febril (contagem baixa de glóbulos brancos e febre) que pode ser causada pelo uso de quimioterapia citotóxica (medicamentos que destroem as células que se dividem rapidamente). Os glóbulos brancos são células importantes porque contribuem para combater as infecções. Estas células são sensíveis aos efeitos da quimioterapia, o que pode fazer com que o seu número diminua. Se o número de glóbulos brancos diminuir muito, pode não haver suficientes para combater as bactérias, o que implica um risco maior de contrair uma infecção.
O seu médico prescreveu Fulphila para estimular a sua medula óssea (a parte do osso onde se produzem as células do sangue) para que produza mais glóbulos brancos que o ajudem a combater as infecções.
Fulphila está indicado em pacientes maiores de 18 anos.
2. O que necessita de saber antes de começar a usar Fulphila
Não use Fulphila
- se é alérgico ao pegfilgrastim, filgrastim ou a algum dos outros componentes deste medicamento (incluídos na secção 6).
Advertências e precauções
Consulte o seu médico, farmacêutico ou enfermeiro antes de começar a usar Fulphila:
- se experimenta uma reação alérgica que inclui fraqueza, diminuição da pressão arterial, dificuldade para respirar, inchaço da face (anafilaxia), vermelhidão e rubor, erupção cutânea e picazón na pele.
- se experimenta tos, febre e dificuldade para respirar. Isto pode ser um sinal do Síndrome de distresse respiratório agudo (SDRA).
- se experimenta algum ou uma combinação dos seguintes efeitos adversos:
- inchaço que pode estar associado a urinar com menor frequência, dificuldade para respirar, inchaço e sensação de plenitude abdominal e uma sensação geral de cansaço.
Estes podem ser sintomas de uma doença chamada “síndrome de fuga capilar” e que pode fazer com que o sangue se escape de pequenos vasos sanguíneos para outros lugares do seu corpo. Ver secção 4.
- se tem dor na parte superior esquerda abdominal ou dor no extremo do ombro. Isto pode ser um sinal de um problema com o baço (esplenomegalia).
- se recentemente teve uma infecção pulmonar grave (pneumonia), líquido nos pulmões (edema pulmonar), inflamação dos pulmões (doença intersticial pulmonar) ou um resultado anormal de raios-X de tórax (infiltração pulmonar).
- se é consciente de alguma alteração na contagem de células sanguíneas (por exemplo, aumento do número de glóbulos brancos ou anemia) ou uma diminuição da contagem de plaquetas sanguíneas, que pode reduzir a capacidade do sangue para coagular (trombocitopenia). O seu médico pode querer realizar-lhe um maior acompanhamento.
- se tem anemia de células falciformes. O seu médico pode supervisionar a sua doença mais estreitamente.
- se é paciente de cancro de mama ou cancro de pulmão, o tratamento combinado de Fulphila com quimioterapia e/ou radioterapia pode aumentar o risco de desenvolver uma doença hematológica precancerosa denominada síndrome mielodisplásico (SMD) ou uma neoplasia hemática denominada leucemia mieloide aguda (LMA). Os sintomas podem incluir cansaço, febre, aparecimento de equimoses com facilidade ou sangramento.
- se tem sinais repentinos de alergia, tais como erupção, picazón ou urticária na pele, inchaço da face, lábios, língua ou outras partes do corpo, falta de ar, sibilância ou dificuldade para respirar; podem ser sinais de uma reação alérgica grave.
- se tem sintomas de inflamação da aorta (o vaso sanguíneo grande que transporta sangue desde o coração até ao resto do corpo) isto raramente se notificou em pacientes com cancro e em doadores sãos. Os sintomas podem incluir febre, dor abdominal, mal-estar geral, dor de costas e marcadores inflamatórios aumentados. Informe o seu médico se sofre estes sintomas.
O seu médico realizará análises de sangue e urina de forma regular, dado que Fulphila pode danificar os pequenos filtros dentro dos rins (glomerulonefrite).
Com o uso de pegfilgrastim, notificaram-se reações cutâneas graves (síndrome de Stevens-Johnson). Deixe de usar Fulphila e procure atendimento médico imediatamente se observar algum dos sintomas descritos na secção 4.
Deve consultar com o seu médico o risco de desenvolver cancro do sangue. Em caso de que apresente ou possa apresentar cancro do sangue, não deve utilizar Fulphila, excepto se o seu médico o aconselhar.
Perda de resposta a Fulphila
Se experimenta uma perda de resposta ou se não se consegue manter a resposta ao tratamento com pegfilgrastim, o seu médico investigará as causas, incluindo se desenvolveu anticorpos que possam neutralizar a atividade de pegfilgrastim.
Crianças e adolescentes
Não se recomenda o uso de Fulphila em crianças e adolescentes, pois não se dispõe de dados suficientes sobre a sua segurança e eficácia.
Outros medicamentos e Fulphila
Informa o seu médico ou farmacêutico se está a utilizar, utilizou recentemente ou pode ter que utilizar qualquer outro medicamento.
Gravidez e amamentação
Se está grávida ou em período de amamentação, acredita que possa estar grávida ou tem intenção de engravidar, consulte o seu médico ou farmacêutico antes de utilizar este medicamento.
Fulphila não foi utilizado em mulheres grávidas. Por isso, o seu médico pode decidir que não deve usar este medicamento.
Se engravidar durante o tratamento com Fulphila, informe o seu médico.
A menos que o seu médico lhe indique o contrário, deve abandonar a amamentação se usar Fulphila.
Condução e uso de máquinas
A influência de Fulphila sobre a capacidade para conduzir e utilizar máquinas é nula ou insignificante.
Fulphila contém sorbitol e sódio
Este medicamento contém 30 mg de sorbitol em cada seringa pré-carregada, o que equivale a 50 mg/ml.
Este medicamento contém menos de 1 mmol de sódio (23 mg) por cada dose de 6 mg, isto é, é essencialmente “isento de sódio”.
3. Como usar Fulphila
Siga exactamente as instruções de administração deste medicamento indicadas pelo seu médico. Em caso de dúvida, consulte novamente o seu médico ou farmacêutico.
A dose recomendada é uma injeção subcutânea de 6 mg (debajo da pele) com uma seringa pré-carregada, e deve ser administrada no final de cada ciclo de quimioterapia a partir das 24 horas após a última dose de quimioterapia.
Auto-injeção de Fulphila
O seu médico pode considerar mais conveniente que se injete Fulphila si mesmo. O seu médico ou enfermeiro ensinar-lhe-á como fazer. Não o tente se não lhe ensinaram.
Para obter mais indicações sobre como se injetar Fulphila si mesmo, leia as instruções anexas. Não agite fortemente Fulphila, pois isso pode afectar a sua actividade.
Se usar mais Fulphila do que deve
Se usar mais Fulphila do que deve, contacte o seu médico, farmacêutico ou enfermeiro.
Se esquecer de usar Fulphila
Se esquecer de administrar a sua dose de Fulphila, contacte o seu médico para decidir quando deve injetar a próxima dose.
Se tiver alguma outra dúvida sobre o uso deste medicamento, pergunte ao seu médico, farmacêutico ou enfermeiro.
4. Possíveis efeitos adversos
Como todos os medicamentos, este medicamento pode produzir efeitos adversos, embora nem todas as pessoas os sofram.
Informa imediatamente o seu médico se experimenta algum ou uma combinação dos seguintes efeitos adversos:
- inchaço que pode estar associado a urinar com menor frequência, dificuldade para respirar, inchaço e sensação de plenitude abdominal e uma sensação geral de cansaço. Estes sintomas geralmente desenvolvem-se muito rapidamente.
Estes podem ser sintomas de uma doença que ocorre de forma pouco frequente (que pode afectar até 1 de cada 100 pessoas) chamada “síndrome de fuga capilar” e que pode fazer com que o sangue se escape de pequenos vasos sanguíneos para outros lugares do seu corpo e necessite de atendimento médico urgente.
Efeitos adversos muito frequentes(podem afectar a mais de 1 de cada 10 pessoas)
- dor óssea. O seu médico informá-lo-á sobre o que pode tomar para aliviar a dor.
- náuseas e cefaleias.
Efeitos adversos frequentes(podem afectar até 1 de cada 10 pessoas)
- dor na zona da injeção.
- dor geral e dor nas articulações e músculos.
- podem ocorrer alguns cambios no seu sangue, que se detectarão mediante análises de sangue periódicas. Pode aumentar o número de glóbulos brancos durante um curto período de tempo. Pode diminuir o número de plaquetas, o que pode provocar a aparecimento de equimoses.
- dor torácica.
Efeitos adversos pouco frequentes(podem afectar até 1 de cada 100 pessoas)
- reações de tipo alérgico, que incluem vermelhidão e rubor, aparecimento de sarpullidos, e inflamação cutânea com picazón.
- reações alérgicas graves, que incluem anafilaxia (fraqueza, queda da pressão arterial, dificuldade para respirar, inchaço facial).
- crises de anemia de células falciformes em pacientes com essa anemia.
- aumento do tamanho do baço.
- ruptura do baço. Alguns casos de ruptura do baço foram mortais. É importante que contacte o seu médico imediatamente se nota dor na parte superior esquerda do abdômen ou no ombro esquerdo, pois isso pode estar relacionado a um problema no baço.
- problemas respiratórios. Se tem tos, febre e dificuldade para respirar, consulte o seu médico.
- produziram-se casos de síndrome de Sweet (lesões dolorosas, inflamadas, de coloração violácea nas extremidades e, por vezes, na face e no pescoço, acompanhadas de febre), mas podem estar relacionados com outros factores.
- vasculite cutânea (inflamação dos vasos sanguíneos cutâneos).
- danos nos pequenos filtros dentro dos rins (glomerulonefrite).
- vermelhidão na zona da injeção.
- tosse com sangue (hemoptise).
- distúrbios hematológicos (SMD ou LMA).
Efeitos adversos raros(podem afectar até 1 de cada 1.000 pessoas)
- inflamação da aorta (o vaso sanguíneo grande que transporta sangue desde o coração até ao resto do corpo), ver secção 2.
- sangramento do pulmão (hemorragia pulmonar).
- síndrome de Stevens-Johnson, que pode aparecer como manchas roxas concêntricas ou circulares, muitas vezes com bolhas centrais no tronco, descamação, úlceras na boca, garganta, nariz, genitais e olhos; e pode ser precedido de febre e sintomas tipo gripal. Deixe de usar Fulphila se desenvolver estes sintomas e contacte o seu médico ou procure atendimento médico imediatamente. Ver secção 2.
Comunicação de efeitos adversos
Se experimenta efeitos adversos, consulte o seu médico, farmacêutico ou enfermeiro, mesmo que se trate de efeitos adversos que não aparecem neste prospecto. Também pode comunicá-los directamente através do sistema nacional de notificação incluído no Apêndice V. Mediante a comunicação de efeitos adversos, pode contribuir para fornecer mais informações sobre a segurança deste medicamento.
5. Conservação de Fulphila
Mantenha este medicamento fora da vista e do alcance das crianças.
Não utilize este medicamento após a data de validade que aparece na caixa, no blister e na etiqueta da seringa após CAD. A data de validade é o último dia do mês que se indica.
Conservar em frigorífico (entre 2 °C e 8 °C).
Não congelar. Fulphila pode ser utilizado em caso de congelamento acidental durante um período inferior a 24 horas.
Conservar o envase no embalagem exterior para protegê-lo da luz.
Fulphila pode estar fora do frigorífico a temperatura ambiente (sempre que não supere os 30 °C) durante um máximo de 3 dias. Uma vez que se retire uma seringa do frigorífico e atinja a temperatura ambiente (que não supere os 30 °C), deve ser utilizado em 3 dias ou deitado fora.
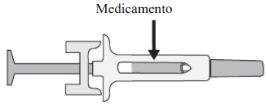
Não utilize este medicamento se observar que a solução não é totally transparente ou contém partículas.
Os medicamentos não devem ser deitados fora por meio dos esgotos nem para o lixo. Pergunte ao seu farmacêutico como deitar fora os envases e os medicamentos que já não precisa. Desta forma, ajudará a proteger o meio ambiente.
6. Conteúdo do envase e informação adicional
Composição de Fulphila
- O princípio ativo é pegfilgrastim. Cada seringa pré-carregada contém 6 mg de pegfilgrastim em 0,6 ml de solução.
- Os outros componentes são acetato de sódio, sorbitol (E420), polissorbato 20 e água para preparações injetáveis. Consulte a seção 2 “Fulphila contém sorbitol e acetato de sódio”.
Aspecto do produto e conteúdo do envase
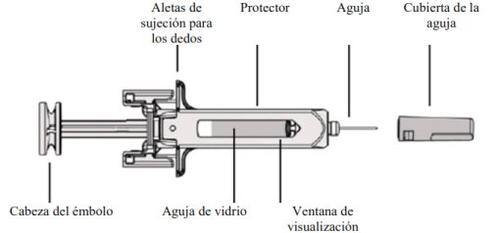
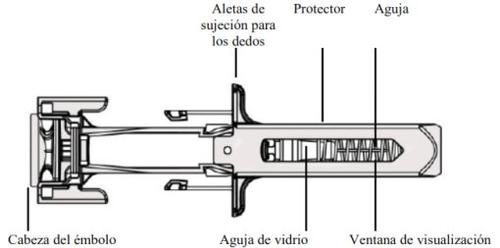
Fulphila é uma solução injetável transparente e incolor (injeção) contida em uma seringa pré-carregada de vidro com uma agulha de aço inoxidável e um capuchão da agulha. A seringa é fornecida embalada em um blister.
Cada envase contém 1 seringa pré-carregada.
Título da autorização de comercialização
Biosimilar Collaborations Ireland Limited
Unidade 35/36
Grange Parade,
Estação Industrial de Baldoyle,
Dublín 13
DUBLÍN
Irlanda
D13 R202
Responsável pela fabricação
Biosimilar Collaborations Ireland Limited
Bloco B, The Crescent Building, Santry Demesne
Dublín
D09 C6X8
Irlanda
Pode solicitar mais informações sobre este medicamento dirigindo-se ao representante local do titular da autorização de comercialização.
Bélgica Biocon Biologics Belgium BV Tel: 0080008250910 | Lituânia Biosimilar Collaborations Ireland Limited Tel: 0080008250910 |
| Luxemburgo Biocon Biologics France S.A.S Tel: 0080008250910 |
República Tcheca Biocon Biologics Germany GmbH Tel: 0080008250910 | Hungria Biosimilar Collaborations Ireland Limited Tel: 0080008250910 |
Dinamarca Biocon Biologics Finland OY Tlf: 0080008250910 | Malta Biosimilar Collaborations Ireland Limited Tel: 00 80008250910 |
Alemanha Biocon Biologics Germany GmbH Tel: 0080008250910 | Países Baixos Biocon Biologics France S.A.S. Tel: 0080008250910 |
Estônia Biosimilar Collaborations Ireland Limited Tel: 0080008250910 | Noruega Biocon Biologics Finland OY Tlf: +47 800 62 671 |
Grécia Biocon Biologics Grécia ΜΟΝΟΠΡΟΣΩΠΗ Ι Κ Ε Tel: 0080008250910 | Áustria Biocon Biologics Germany GmbH Tel: 0080008250910 |
Espanha Biocon Biologics Espanha S.L. Tel: 0080008250910 | Polônia Biosimilar Collaborations Ireland Limited Tel: 0080008250910 |
França Biocon Biologics France S.A.S Tel: +Teél: 0080008250910 | Portugal Biocon Biologics Espanha S.L.. Tel: 0080008250910 |
Croácia Biocon Biologics Germany GmbH Tel: 0080008250910 | Romênia Biosimilar Collaborations Ireland Limited Tel: 0080008250910 |
Irlanda Biosimilar Collaborations Ireland Limited Tel: 1800 777 794 | Eslovênia Biosimilar Collaborations Ireland Limited Tel: 0080008250910 |
Islândia Biocon Biologics Finland OY Sími: +345 8004316 | República Eslovaca Biocon Biologics Germany GmbH Tel: 0080008250910 |
Itália Biocon Biologics Espanha S.L. Tel: 0080008250910 | Finlândia Biocon Biologics Finland OY Puh/Tel: 99980008250910 |
Chipre Biosimilar Collaborations Ireland Limited Tel: 0080008250910 | Suécia Biocon Biologics Finland OY Tel: 0080 008250910 |
Letônia Biosimilar Collaborations Ireland Limited Tel: 0080008250910 |
Data da última revisão deste prospecto: {MM/AAAA}.
Outras fontes de informação
A informação detalhada deste medicamento está disponível no site da Agência Europeia de Medicamentos: http://www.ema.europa.eu.
---------------------------------------------------------------------------------------------------------------------
Instruções de uso: |
Guia dos componentes |
Antes de usar |
|
Depois de usar |
|
Importante | ||
|
Paso 1: Preparação | |
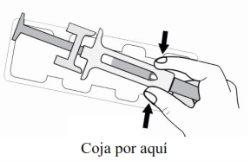
A. | Retire a bandeja da seringa pré-carregada que se encontra no interior do envase e pegue os materiais que necessite para a injeção: gaze com álcool, algodão ou gaze, bandagens e um contenedor de objetos pontiagudos (não incluído). |
Para uma injeção menos dolorosa, deixe a seringa pré-carregada à temperatura ambiente durante aproximadamente 30 minutos antes da injeção. Lave bem as mãos com água e sabão. Coloque a seringa pré-carregada nova e os outros materiais sobre uma superfície limpa e bem iluminada.
|
B. | Abra o envase, retirando a cobertura. Pegue a seringa pré-carregada pelo protetor de segurança para sacá-la da bandeja. |
Por motivos de segurança:
|
C. | Examine o medicamento e a seringa pré-carregada. |
Em qualquer um desses casos, entre em contato com o seu médico ou profissional de saúde. |
Paso 2: Prepare-se | |
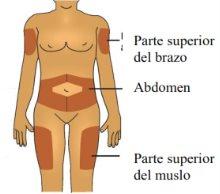
A. | Lave bem as mãos. Prepare e limpe o local da injeção. |
Pode injetar o medicamento em:
Limpe o local da injeção com uma gaze com álcool. Deixe a pele secar.
Não se injete em áreas onde a pele esteja sensível, contundida, vermelha ou com endurecimentos. Evite se injetar em áreas com cicatrizes ou estrias. |
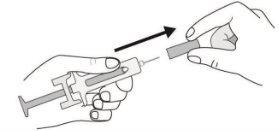
B | Tire cuidadosamente o capuchão gris da agulha em linha reta, mantendo a seringa separada do seu corpo. |
|
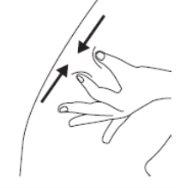
C | Pegue o local da injeção para criar uma superfície firme. |
É importante manter a pele pega quando se injetar. |
Paso 3: Injete | |
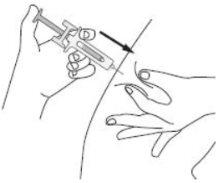
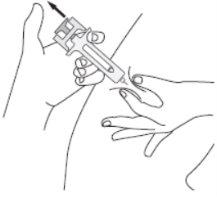
A | Mantenha a pele pega. INSIRA a agulha na pele. |
|
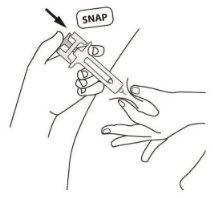
B | EMPURRE a cabeça do êmbolo com uma pressão leve e constante até sentir ou ouvir um “clic”. Empurre completamente para baixo até ouvir o “clic”. |
É importante pressionar para baixo até ouvir o “clic” para receber toda a dose. |
C | DEIXE DE PRESSIONAR a cabeça do êmbolo. Em seguida, SEPARE a seringa da pele. |
Depois de soltar a cabeça do êmbolo, o protetor de segurança da seringa pré-carregada cobrirá de forma segura a agulha.
|
Somente para profissionais de saúde A marca comercial do produto administrado deve estar corretamente registrada na história clínica do paciente. |
Paso 4: Final | |
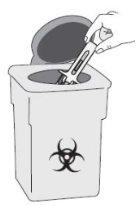
A | Descarte a seringa pré-carregada usada e outros materiais em um contenedor para descartar objetos pontiagudos. |
Os medicamentos devem ser eliminados de acordo com a regulamentação local. Pergunte ao seu farmacêutico como se livrar dos envases e dos medicamentos que já não precisa. Dessa forma, ajudará a proteger o meio ambiente. Mantenha a seringa e o contenedor de objetos pontiagudos fora da vista e do alcance das crianças.
|
B | Examine o local da injeção. |
Se observar sangue, pressione com um algodão ou uma gaze no local da injeção. Não esfregue o local da injeção. Coloque uma bandagem se necessário. |
- País de registo
- Substância ativa
- Requer receita médicaSim
- Fabricante
- Esta informação é apenas para referência e não constitui aconselhamento médico. Consulte sempre um médico antes de tomar qualquer medicamento. A Oladoctor não se responsabiliza por decisões médicas baseadas neste conteúdo.
- Alternativas a FULPHILA 6 mg SOLUÇÃO INJETÁVEL EM SERINGA PREENCHIDAForma farmacêutica: INJETÁVEL, 6 mg de pegfilgrastim (10mg/ml)Substância ativa: pegfilgrastimFabricante: Amgen Europe B.V.Requer receita médicaForma farmacêutica: INJETÁVEL, 6 mgSubstância ativa: pegfilgrastimFabricante: Amgen Europe B.V.Requer receita médicaForma farmacêutica: INJETÁVEL, 6 mgSubstância ativa: pegfilgrastimFabricante: Pfizer Europe Ma EeigRequer receita médica
Alternativas a FULPHILA 6 mg SOLUÇÃO INJETÁVEL EM SERINGA PREENCHIDA noutros países
As melhores alternativas com o mesmo princípio ativo e efeito terapêutico.
Alternativa a FULPHILA 6 mg SOLUÇÃO INJETÁVEL EM SERINGA PREENCHIDA em Украина
Médicos online para FULPHILA 6 mg SOLUÇÃO INJETÁVEL EM SERINGA PREENCHIDA
Avaliação de posologia, efeitos secundários, interações, contraindicações e renovação da receita de FULPHILA 6 mg SOLUÇÃO INJETÁVEL EM SERINGA PREENCHIDA – sujeita a avaliação médica e regras locais.