FLOLAN 0,5 mg PÓ E SOLVENTE PARA SOLUÇÃO PARA PERFUSÃO

Pergunte a um médico sobre a prescrição de FLOLAN 0,5 mg PÓ E SOLVENTE PARA SOLUÇÃO PARA PERFUSÃO

Como usar FLOLAN 0,5 mg PÓ E SOLVENTE PARA SOLUÇÃO PARA PERFUSÃO
Introdução
Prospecto: informação para o utilizador
Flolan 0,5 mg pó e dissolvente para solução para perfusão
Epoprostenol
Leia todo o prospecto atentamente antes de começar a usar este medicamento,porque contém informações importantes para si.
- Conserva este prospecto, porque pode ter que voltar a lê-lo.
- Se tiver alguma dúvida, consulte o seu médico, farmacêutico ou enfermeiro.
- Este medicamento foi-lhe prescrito apenas para si, e não deve dá-lo a outras pessoas
embora tenham os mesmos sintomas que si, porque pode prejudicá-las.
- Se experimentar efeitos adversos, consulte o seu médico, farmacêutico ou enfermeiro, mesmo que se trate de efeitos adversos que não aparecem neste prospecto. Ver secção 4.
Conteúdo do prospecto
- O que é Flolan e para que é utilizado
- O que necessita saber antes de começar a usar Flolan
- Como usar Flolan
- Possíveis efeitos adversos
- Conservação de Flolan
- Conteúdo do envase e informação adicional
1. O que é Flolan e para que é utilizado
O que é Flolan
Flolan contém o princípio ativo epoprostenol, que pertence a um grupo de medicamentos denominados prostaglandinas que evita a coagulação do sangue e dilata os vasos sanguíneos.
Para que é utilizado Flolan
- Flolan é utilizado para tratar uma doença do pulmão conhecida como “hipertensão arterial pulmonar”. Esta ocorre quando a pressão dos vasos sanguíneos no pulmão é alta. Flolan dilata os vasos sanguíneos para diminuir a pressão do sangue nos pulmões.
- Flolan é utilizado para prevenir a coagulação do sangue durante a diálise renal, em situações de emergência, quando a heparina não pode ser utilizada.
2. O que necessita saber antes de começar a usar Flolan
Não use Flolan
- se é alérgicoa epoprostenol ou a algum dos outros componentes deste medicamento (incluídos na secção 6)
- se tem uma insuficiência cardíaca.
- se começa a desenvolver uma acumulação de líquido nos pulmões causando dificuldade para respirar após o início do tratamento.
Se considera que lhe aplica alguma destas situações, não useFlolanaté que tenha consultado o seu médico.
Advertências e precauções
Informa o seu médico antes de usar Flolan:
- se tem problemas de sangramento
- se está a realizar uma dieta baixa em sódio.
Lesão na pele no local da injeção
Flolan é injetado numa veia. É importante que o medicamento não saia ou filtre para fora da veia para os tecidos ao redor. Se o fizer, danificará a pele. Os sintomas são:
- sensibilidade
- queimadura
- picazón
- inchação
- vermelhidão.
Isso pode continuar com a formação de bolhas e descamação da pele. É importante que controle a área da injeção enquanto está a ser tratado com Flolan.
Entre em contacto imediatamente com o hospitalse a zona da injeção se tornar dolorosa, arder ou inflamar ou notar qualquer formação de bolhas ou descamação.
Efeito de Flolan sobre a pressão sanguínea e a frequência cardíaca
Flolan pode acelerar ou desacelerar o batimento do coração. Também a sua pressão sanguínea pode tornar-se muito baixa. Enquanto está a ser tratado com Flolan, a sua frequência cardíaca e a sua pressão sanguínea devem ser controladas. Os sintomas de uma pressão sanguínea baixa incluem tonturae desmaio.
Informa o seu médicose tiver algum destes sintomas. Pode ser necessário diminuir a sua dose ou interromper a perfusão.
Uso de Flolancomoutros medicamentos
Informa o seu médico ou farmacêutico se está a tomar, tomou recentemente ou pode ter que tomar qualquer outro medicamento, mesmo os adquiridos sem receita médica.
Alguns medicamentos podem afetar o funcionamento de Flolan, ou fazer com que seja mais provável que aumentem os seus efeitos adversos. Flolan também pode afetar o funcionamento de outros medicamentos se forem tomados ao mesmo tempo. Entre estes incluem-se:
- medicamentos utilizados para tratar a pressão sanguínea alta
- medicamentos utilizados para evitar a formação de coágulos no sangue
- medicamentos utilizados para dissolver os coágulos do sangue
- medicamentos utilizados para tratar a inflamação ou a dor(também chamados AINEs)
- digoxina (utilizada para o tratamento de distúrbios cardíacos).
Comunique ao seu médico ou farmacêuticose está a tomar algum destes medicamentos.
Gravidez elactação
Se está grávida ou em período de lactação, acredita que possa estar grávida ou tem intenção de ficar grávida, consulte o seu médico ou farmacêutico antes de utilizar este medicamento, porque os sintomas podem piorar durante a gravidez.
Desconhece-sese os componentes de Flolan podem passar para o leite humano. Deve interromper a lactação enquanto está a ser tratada com Flolan.
Condução e uso de máquinas
O tratamento pode afetar a capacidade para conduzir e usar máquinas.
Não conduza ou use máquinasa menos que se sinta bem.
Flolan contém sódio (componente principal do sal de mesa/para cozinhar)
Os pacientes com dietas pobres em sódio devem ter em conta que este medicamento reconstituído contém 73 mg de sódio por dose.
Solução concentrada reconstituída:este medicamento contém 73 mg de sódio (componente principal do sal de mesa/para cozinhar) em cada frasco de solução concentrada. Isto equivale a 4% da ingestão diária máxima de sódio recomendada para um adulto.
Pó para solução para perfusão:este medicamento contém 3 mg de sódio (componente principal do sal de mesa/para cozinhar) em cada frasco de pó para solução. Isto equivale a 0,2% da ingestão diária máxima de sódio recomendada para um adulto.
Disolvente para uso parenteral:este medicamento contém 70 mg de sódio (componente principal do sal de mesa/para cozinhar) em cada frasco de disolvente. Isto equivale a 4% da ingestão diária máxima de sódio recomendada para um adulto.
3. Como usar Flolan
Siga exatamente as instruções de administração deste medicamento indicadas pelo seu médico ou farmacêutico. Em caso de dúvida, consulte novamente o seu médico ou farmacêutico.
O seu médico indicar-lhe-á quanto Flolan é conveniente para si. A quantidade que lhe é administrada está baseada no seu peso corporal e no tipo de doença. A sua dose pode ser aumentada ou diminuída dependendo de como responde ao tratamento.
Flolan é administrado mediante perfusão lenta (gotejamento) numa veia.
Hipertensão arterial pulmonar
O primeiro tratamento será administrado num hospital. Isto é devido ao facto de o médico precisar monitorizá-lo e identificar a melhor dose para si.
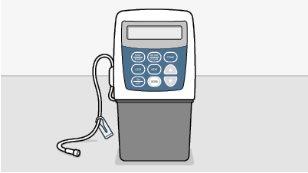
Irão começar com uma perfusão de Flolan. Irão aumentar a dose, até que os seus sintomas se aliviem e os efeitos adversos sejam controlados. Uma vez que a melhor dose tenha sido identificada, será colocada uma via permanente (catéter) numa das suas veias. Em seguida, poderá ser tratado mediante uma bomba para perfusão.
Diálise renal
Ser-lhe-á administrada uma perfusão de Flolan durante a duração da sua diálise.
Uso de Flolan em casa (apenas para o tratamento de hipertensão arterial pulmonar)
Se está a ser tratado em casa, o seu médico ou enfermeiro ensinar-lhe-á como deve preparar e usar Flolan. Também lhe indicará como deve interromper o tratamento se for necessário. A interrupção de Flolan deve ser feita gradualmente. É importante que siga cuidadosamente todasas instruções.
Flolan vem como um pó num frasco de vidro. Antes de usar, o pó precisa ser dissolvido no líquido fornecido. O líquido não contém conservantes. Deverá deitar fora qualquer líquido que não utilize.
Cuidado com o catéter
Se lhe foi colocado um catéter numa veia, é muito importantemanter esta área limpa, senão pode infectar-se. O seu médico ou enfermeiro ensinar-lhe-á como limpar o catéter e a área ao redor dele. É muito importante que siga cuidadosamente todas as instruções.
Se usar mais Flolan do que deve
Procure atendimento médico urgentese pensa que usou ou lhe foi administrado demasiado Flolan. Os sintomas de sobredose podem incluir dor de cabeça, náuseas, vómitos, batimento rápido do coração, calor ou formigamento ou sensação de que pode desmaiar (sensação de tontura/vertigem).
Se esquecer de usar Flolan
Não use uma dose dupla para compensar as doses esquecidas.
Se interromper o tratamento com Flolan
A interrupção de Flolan deve ser feita gradualmente. Se interromper o tratamento demasiado rapidamente, pode ter efeitos adversos graves, incluindo tontura, sensação de fraqueza e dificuldade para respirar. Se tiver problemas com a bomba de perfusão ou o catéter, porque se detém ou impede o tratamento com Flolan, entre em contacto imediatamentecom o seu médico, enfermeiro ou hospital.
Se tiver alguma outra dúvida sobre o uso deste medicamento, pergunte ao seu médico, farmacêutico ou enfermeiro.
4. Possíveis efeitos adversos
Como todos os medicamentos, este medicamento pode produzir efeitos adversos, embora nem todas as pessoas os sofram.
Informa o seu médico ou enfermeiro imediatamente, porque estes podem ser sinais de infeção do sangue ou pressão sanguínea baixa ou hemorragias graves:
- Sente que o seu coração bate mais rápido, ou que tem dor no peito ou dificuldade para respirar.
- Sente que está tonto ou sente desfalecimento, especialmente estando de pé.
- Tem febre ou arrepios.
- Sangra com mais frequência ou durante períodos mais prolongados.
Se experimentar efeitos adversos, consulte o seu médico, farmacêutico ou enfermeiro, mesmo que se trate de efeitos adversos que não aparecem neste prospecto.
Efeitos adversos muito frequentes
Estes podem afetar a mais de 1 em cada 10pessoas:
- dor de cabeça
- dor de mandíbula
- dor inespecífica
- vómitos
- náuseas
- diarreia
- rubor facial.
Efeitos adversos frequentes
Estes podem afetar até 1 em cada 10pessoas:
- infeção do sangue (septicemia)
- batimentos rápidos do coração
- batimentos lentos do coração
- pressão sanguínea baixa
- sangramento em diversas localizações e maior facilidade de aparecimento de hematomas; por exemplo, sangramento pelo nariz ou gengivas
- mal-estar ou dor de estômago
- dor no peito
- dor nas articulações
- sensação de ansiedade, sensação de nervosismo
- erupção na pele
- dor no local da injeção.
Efeitos adversos frequentesque podem aparecer nos seus análises de sangue
- diminuição do número de plaquetas no sangue (células que ajudam à coagulação sanguínea).
Efeitos adversos pouco frequentes
Estes podem afetar até 1 em cada 100pessoas:
- suor
- secura da boca.
Efeitos adversos raros
Estes podem afetar até 1 em cada 1.000pessoas:
- infeção no local da injeção.
Efeitos adversos muito raros
Estes podem afetar até 1 em cada 10.000pessoas:
- sensação de opressão ao redor do peito
- cansaço, fraqueza
- agitação
- palidez
- rubor no local da injeção
- glândulas tiroideias hiperativas
- oclusão do catéter intravenoso.
Outros efeitos adversos
Não se conhece quantas pessoas estão afetadas:
- baço aumentado ou hiperativo
- acumulação de líquido nos pulmões (edema pulmonar)
- aumento de açúcar (glicose) no sangue
- inchação devida à acumulação de líquidos ao redor do estômago
- bombagem excessiva de sangue desde o coração que dá lugar a respiração dificultosa, fadiga, inchação das pernas e do abdômen devido à retenção de líquidos, tosse persistente.
Comunicação de efeitos adversos
Se experimentar qualquer tipo de efeito adverso, consulte o seu médico, farmacêutico ou enfermeiro, mesmo que se trate de possíveis efeitos adversos que não aparecem neste prospecto.
Também pode comunicá-los directamente através do Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano, www.notificaRAM.es. Mediante a comunicação de efeitos adversos, você pode contribuir para proporcionar mais informações sobre a segurança deste medicamento.
5. Conservação de Flolan
Mantenha este medicamento fora da vista e do alcance das crianças.
Não use este medicamento após a data de validade que aparece na etiqueta.
Não conserve acima de 25ºC.
Conserva Flolan num local seco.
Conserva no estojo original para protegê-lo da luz.
Não congele.
Hipertensão arterial pulmonar
Para soluções ≤ 150.000 ng/ml:
A solução recém-preparada de Flolan (seja como solução concentrada ou mais diluída) pode ser administrada imediatamente ou armazenada durante um máximo de 8 dias na geladeira (entre 2ºC e 8ºC), no cartucho de medicação e utilizada dentro de um prazo máximo de:
- 72 horas até os 25ºC ou
- 48 horas até os 30ºC ou
- 24 horas até os 35ºC ou
- 12 horas até os 40ºC.
Para soluções > 150.000 ng/ml e ≤ 300.000 ng/ml:
As soluções reconstituídas que sejam armazenadas a 2-8°C durante até 7 dias podem ser administradas até 24 horas a 25°C.
As soluções reconstituídas recém-preparadas ou aquelas soluções que tenham sido armazenadas a 2-8°C durante não mais de 5 dias podem ser administradas até:
- 48 horas até os 25ºC
- 24 horas até os 35ºC
Elimine qualquer solução não utilizada após esse tempo.
Diálise renal
Uma vez que Flolan seja dissolvido e diluído, qualquer solução que não seja utilizada pode ser armazenada a 25ºC e usada nas 12 horas seguintes.
Os medicamentos não devem ser jogados nos esgotos nem na lixeira. Deposite os recipientes e os medicamentos que não precisa no Ponto SIGRE  da farmácia. Em caso de dúvida, pergunte ao seu farmacêutico como se livrar dos recipientes e dos medicamentos que não precisa. Dessa forma, ajudará a proteger o meio ambiente.
da farmácia. Em caso de dúvida, pergunte ao seu farmacêutico como se livrar dos recipientes e dos medicamentos que não precisa. Dessa forma, ajudará a proteger o meio ambiente.
6. Conteúdo do envase e informação adicional
Composição de Flolan
O princípio ativo é epoprostenol de sódio.
Cada frasco contém:
- 0,5 mg de epoprostenol de sódio
- Ver seção 2 para mais informações importantes sobre o sódio.
Os outros componentes são manitol, glicina, cloreto de sódio, hidróxido de sódio e água para preparações injetáveis.
Aspecto do produto e conteúdo do envase
Injeção:
Flolan é uma solução injetável composta de pó e dissolvente. O pó é de cor branca a esbranquiçada e a solução é transparente e incolor.
Há três envases de Flolan disponíveis para o uso em hipertensão arterial pulmonar, o conteúdo de cada caixa inclui:
- Um frasco de 0,5 mg de pó e um frasco de dissolvente, um adaptador de frasco e um filtro.
- Um frasco de 0,5 mg de pó e dois frascos de dissolvente, dois adaptadores de frasco e um filtro.
- Um frasco de 0,5 mg de pó.
Há apenas um envase de Flolan disponível para uso em diálise renal, o conteúdo de cada caixa inclui:
- Um frasco de 0,5 mg de pó e um frasco de dissolvente, um adaptador de frasco e um filtro.
Pode ser que apenas alguns tamanhos de envases sejam comercializados.
Titular da autorização de comercialização e responsável pela fabricação
Titular da autorização de comercialização:
GlaxoSmithKline, S.A.
P.T.M. C/ Severo Ochoa, 2
28760 Tres Cantos (Madrid)
Tel: +34 900 202 700
Responsável pela fabricação:
GlaxoSmithKline Manufacturing, S.p.A.
Strada Provinciale Asolana, 90
43056 San Polo di Torrile, Parma, Itália.
ou
GlaxoSmithKline Pharmaceuticals S.A.
Ul. Grunwaldzka
60-322 Poznan
Polônia.
ou
Glaxo Wellcome GmbH & Co.KG
Industriestrasse 32-36
23843 Bad Oldesloe
Alemanha.
Este medicamento está autorizado nos estados membros do Espaço Econômico Europeu com os seguintes nomes:
Áustria Flolan
Bélgica Flolan
República Checa Flolan
Dinamarca Epoprostenol
Estônia Flolan
França Flolan
Irlanda Flolan
Itália Flolan
Luxemburgo Flolan
Malta Flolan
Holanda Flolan
Noruega Flolan
Espanha Flolan
Reino Unido Flolan
Data da última revisão desteprospecto: março 2021
A informação detalhada e atualizada deste medicamento está disponível na página Web da Agência Espanhola de Medicamentos e Produtos Sanitários (AEMPS) http://www.aemps.gob.es/
-------------------------------------------------------------------------------------------------------------------------
Esta informação está destinada apenas a médicos ou profissionais do setor sanitário:
- INFORMAÇÃO PARA OS PROFISSIONAIS SANITÁRIOS
Hipertensão arterial pulmonar
Há três envases disponíveis para o uso em hipertensão arterial pulmonar:
- Um frasco de 0,5 mg de pó, um frasco de dissolvente, um adaptador de frasco e um filtro.
- Um frasco de 0,5 mg de pó, dois frascos de dissolvente, dois adaptadores de frasco e um filtro.
- Um frasco de 0,5 mg de pó.
Pode ser que apenas alguns tamanhos de envases sejam comercializados.
Inicialmente, deve-se utilizar um envase que contenha dissolvente para uso parenteral. Pode ser que sejam necessárias soluções mais concentradas durante o tratamento crônico de Flolan. A solução concentrada final pode ser aumentada mediante a adição de outro frasco de pó liofilizado de 0,5 mg de Flolan.
Para aumentar a concentração final da solução, apenas devem ser utilizados frascos da mesma quantidade de Flolan pó liofilizado que os incluídos no envase inicial.
Flolan preparado com dissolvente (pH 11,7-12,3) não deve ser utilizado com nenhuma preparação ou material de administração que contenha polietileno tereftalato (PET) ou polietileno tereftalato modificado com glicol (PETG). Com base nos dados disponíveis dos análises internos e da literatura publicada, os materiais de preparação e administração compatíveis incluem:
- Acrílico modificado
- Acetonitrilo butadieno estireno (ABS)
- Polímero de olefina cíclica
- Poliamida
- Poliétersulfona
- Polietileno
- Poliisopreno
- Poliolefina
- Polipropileno
- Politetrafluoroetileno (PTFE)
- Poliuretano
- Cloreto de polivinilo (PVC) (plastificado com bis(2-etilhexil) ftalato (DEHP)
- Polivinilideno fluoruro (PVDF)
- Silicona
As bombas ambulatorias adequadas para o uso incluem:
- CADD- Legacy 1
- CADD- Legacy PLUS
- CADD-Solis VIP (perfil de perfusão variável)
Fabricadas por Smiths Medical.
Os acessórios das bombas que são compatíveis incluem:
- Cassete de medicação CADD “reservoir” 50 ml; e 100 ml de Smiths Medical.
- Conjunto de extensão CADD com filtro em linha de 0,2 micras (conjunto de extensão CADD com luer macho; filtro de eliminação de ar de 0,2 micras, abrazadeira e válvula antisifão integral com luer macho) de Smiths Medical.
O conjunto de extensão e o filtro em linha devem ser trocados pelo menos a cada 48 horas.
Reconstituição:
- Utilize apenas o dissolvente fornecido para a reconstituição.
- Retire aproximadamente 10 ml do dissolvente com uma seringa estéril, através de um adaptador de frasco*.
- Retire a seringa do adaptador de frasco. Conecte a agulha à seringa, injete os 10 ml de dissolvente dentro do frasco que contém Flolan em pó e agite suavemente até que o pó se dissolva.
- Extraia a solução resultante de Flolan na seringa, retire a agulha, reinjete-a no volume restante do dissolvente através do adaptador de frasco* e misture bem.
*Alternativamente, pode-se usar uma agulha em vez do adaptador de frasco
A esta solução agora se chama solução concentrada e contém 10.000 nanogramas por ml de Flolan. Apenas as soluções concentradas são adequadas para uma posterior diluição antes do uso. Use um novo adaptador de frasco para cada frasco adicional de dissolvente estéril que necessite. Quando 0,5 mg de Flolan em pó se reconstituem com 50 ml do dissolvente, a injeção final terá um pH aproximado de 12 e um conteúdo de íon sódio de aproximadamente 73 mg.
Diluição:
Pode-se utilizar Flolan em solução concentrada ou em forma diluída para o tratamento da hipertensão arterial pulmonar. Apenas as soluções concentradas são adequadas para diluições adicionais com o dissolvente estéril antes do uso. Apenas o dissolvente fornecido pode ser utilizado na diluição posterior de Flolan reconstituído, usando um novo adaptador de frasco para cada frasco adicional de dissolvente estéril que seja necessário.
A solução de cloreto de sódio a 0,9% p/v não deve ser utilizada se for administrar Flolan para o tratamento da hipertensão arterial pulmonar, pois não se mantém o pH necessário. As soluções de Flolan são menos estáveis em pH baixo.
Epoprostenol não deve ser administrado com outras soluções ou medicações parenterais quando for administrado na hipertensão arterial pulmonar.
A solução final que será administrada ao paciente deve ser filtrada usando um filtro de 0,22 ou 0,20 micras. É preferível o uso de um filtro em linha como parte do equipamento de perfusão durante a administração. Alternativamente, quando não for possível a filtragem em linha, a solução final (seja como solução concentrada ou mais diluída) deve ser filtrada com o filtro estéril de 0,22 micras fornecido antes de ser armazenada no cassete de medicação, exercendo uma pressão firme mas não excessiva. O tempo habitual para a filtragem de 50 ml de solução é de 70 segundos.
Se um filtro em linha for utilizado durante a administração, deve ser descartado ao trocar o equipamento de perfusão.
Se, em vez disso, um filtro de seringa for utilizado durante a preparação, deve ser usado apenas para a preparação e descartado após.
As concentrações habitualmente utilizadas no tratamento da hipertensão arterial pulmonar são as seguintes:
- 5.000 nanogramas/ml – um frasco que contém 0,5 mg de Flolan reconstituído e diluído a um volume total de 100 ml no dissolvente.
- 10.000 nanogramas/ml - dois frascos que contêm 0,5 mg de Flolan reconstituído e diluído a um volume total de 100 ml no dissolvente.
Cálculo da velocidade de perfusão:
A velocidade de perfusão pode ser calculada a partir da seguinte fórmula:
dose (nanogramas/kg/min) x peso corporal (kg)
Velocidade de perfusão (ml/min) =
concentração da solução (nanogramas/ml)
Velocidade de perfusão (ml/h) = velocidade de perfusão (ml/min) x 60
Na administração de Flolan a longo prazo, podem ser necessárias velocidades de perfusão maiores, e portanto, soluções mais concentradas.
Precauções especiais de conservação
Não conservar acima de 25°C.
Conservar o envase dentro da caixa para protegê-lo da luz.
Mantenha em um local seco.
Não congelar.
Para mais detalhes sobre a estabilidade após a reconstituição, consulte a seção 5 (conservação de Flolan).
O dissolvente não contém conservantes; portanto, cada frasco deve ser utilizado apenas uma vez e descartado posteriormente.
Diálise renal
Apenas há um envase disponível para o uso em diálise renal:
- Um frasco de 0,5 mg de pó e um frasco de dissolvente, um adaptador de frasco e um filtro.
Flolan preparado com dissolvente estéril (pH 12) não deve ser utilizado com nenhuma preparação ou material de administração que contenha polietileno tereftalato (PET) ou polietileno tereftalato modificado com glicol (PETG). Com base nos dados disponíveis dos análises internos e da literatura publicada, os materiais de preparação e administração provavelmente compatíveis incluem:
- Acrílico modificado
- Acetonitrilo butadieno estireno (ABS)
- Polímero de olefina cíclica
- Poliamida
- Poliétersulfona
- Polietileno
- Poliisopreno
- Poliolefina
- Polipropileno
- Politetrafluoroetileno (PTFE)
- Poliuretano
- Cloreto de polivinilo (PVC) (plastificado com bis(2-etilhexil) ftalato (DEHP)
- Polivinilideno fluoruro (PVDF)
- Silicona
Reconstituição:
- Utilize apenas o dissolvente fornecido para a reconstituição.
- Retire aproximadamente 10 ml de dissolvente em uma seringa estéril, através de um adaptador de frasco*.
- Retire a seringa do adaptador de frasco. Conecte a agulha à seringa, injete os 10 ml de dissolvente no frasco que contém os 0,5 mg de pó liofilizado de Flolan e agite suavemente até que o pó se dissolva.
- Extraia a solução resultante de Flolan na seringa, retire a agulha, reinjete-a no volume restante de dissolvente, através do adaptador de frasco*, e misture bem.
*Alternativamente, pode-se usar uma agulha em vez do adaptador de frasco.
A esta solução agora se chama solução concentrada e contém 10.000 nanogramas por ml de Flolan. Apenas esta solução concentrada é adequada para posterior diluição antes do uso. Quando 0,5 mg de Flolan em pó para perfusão intravenosa forem reconstituídos com 50 ml do dissolvente, a injeção final terá um pH aproximado de 12 e um conteúdo de íon sódio de aproximadamente 73 mg.
Diluição:
Normalmente, a solução concentrada é diluída novamente imediatamente antes do uso. Pode ser diluída com uma solução de cloreto de sódio 0,9% p/v (solução salina), em uma proporção de 2,3 volumes de solução salina para 1 volume de solução concentrada; por exemplo, 50 ml de solução concentrada diluída posteriormente com 117 ml de solução salina.
Não são convenientes outros fluidos i.v. comuns para a diluição da solução concentrada, pois não se manteria o pH requerido. As soluções de Flolan são menos estáveis em pH baixo.
Para diluir a solução concentrada, extraia-a com uma seringa maior e
administre a solução concentrada diretamente na solução de perfusão escolhida. Misture bem.
A solução final para a perfusão (seja como solução concentrada ou mais diluída) deve ser transferida para um recipiente ou sistema de administração adequado antes da administração. Durante a transferência, deve-se utilizar um filtro de seringa estéril de 0,22 micras, exercendo uma pressão firme mas não excessiva. O tempo habitual para a filtragem de 50 ml de solução é de 70 segundos.
O filtro de seringa deve ser utilizado apenas durante a preparação e, posteriormente, descartado.
Se reconstituído e diluído como descrito anteriormente, as soluções de perfusão de Flolan manterão 90% de sua potência inicial durante cerca de 12 horas a 25°C.
Cálculo da velocidade de perfusão:
A velocidade de perfusão pode ser calculada a partir da seguinte fórmula:
dose (nanogramas/kg/min) x peso corporal (kg)
Velocidade de perfusão (ml/min) =
concentração da solução (nanogramas/ml)
Velocidade de perfusão (ml/h) = velocidade de perfusão (ml/min) x 60
Para a administração mediante uma bomba capaz de fornecer perfusões constantes de pequeno volume, podem ser diluídas alíquotas adequadas da solução concentrada com uma solução estéril de cloreto de sódio a 0,9% p/v.
A informação detalhada e atualizada deste medicamento está disponível na página Web da Agência Espanhola de Medicamentos e Produtos Sanitários (AEMPS) http://www.aemps.gob.es/
INSTRUÇÕES DE USO

Flolan Solução
Instruções de uso com um adaptador de frasco e bombas de perfusão ambulatorias
Por favor, leia estas instruções antes de começar a preparar sua solução de Flolan. Se tiver alguma dúvida ou pergunta, entre em contato com seu médico.
- Lave minuciosamente as mãos antes de tocar os elementos necessários.
- Coloque seus luvas antes da “Preparação” de Flolan (Passo 1).
- Mantenha sua área de trabalho e os elementos necessários para a preparação de Flolan limpos e secos para garantir que prepare Flolan de maneira higiênica.
- Siga sempre as instruções de seu médico de maneira exata. A informação que aparece nestas instruções de uso serve como lembrete do processo.
Informação de conservação
- Mantenha fora da vista e do alcance das crianças.
- Conservar Flolan em um local fresco e seco.
- Proteger da luz mantendo Flolan em sua caixa até que seja usado.
Nãoutilize Flolan após a data de validade que aparece na etiqueta.
Nãocongelar.

Seu envase conterá o seguinte: |
Ou
Ou
|
Também necessitará (não fornecido): |
1 seringa de 60 ml
|
Preparação | |
| |
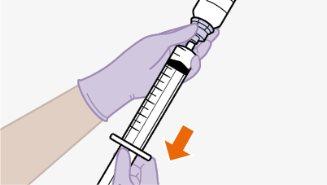
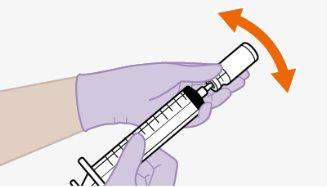
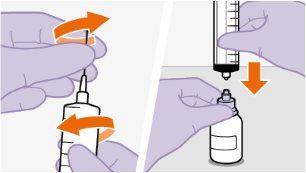
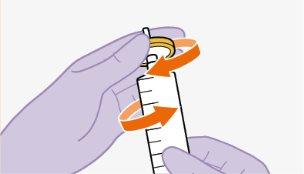
| Use apenas o dissolvente fornecido para a reconstituição.
|
| |
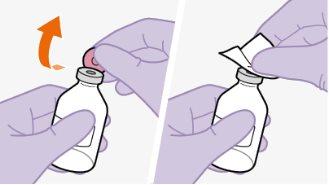
|
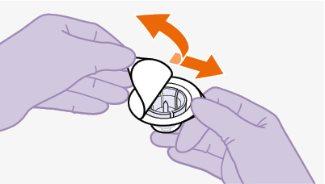
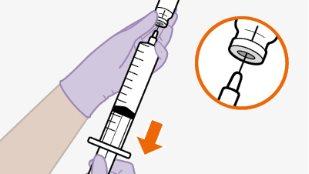
Nota:mantenha o adaptador em seu lugar dentro do envase para o próximo passo. Nãoutilize o adaptador de frasco se o envase estiver danificado. Entre em contato com seu médico ou farmacêutico para mais informações. Nãoutilize o adaptador de frasco se ele sair do envase. |
| |
|
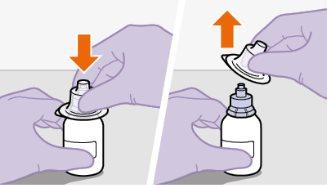
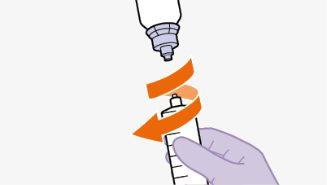
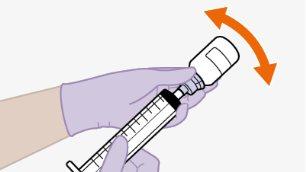
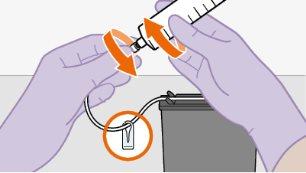
toalheta com álcool. Nãotoque a ponta do adaptador nem a seringa. |
| ||||||||||||||||||||||||||||||||||||||||||||
|
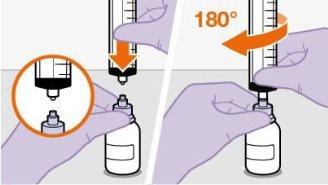
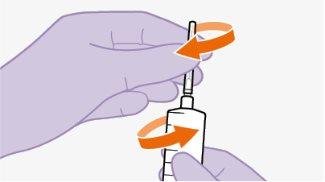
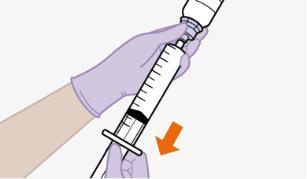
Nãoinsira a agulha na seringa. | |||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||
|
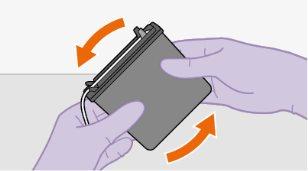
| |||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||
|
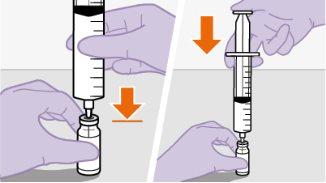
Nota:se preparar uma s...
|