
ESCITALOPRAM SANDOZ 15 mg COMPRIMIDOS ORODISPERSÍVEIS

Pergunte a um médico sobre a prescrição de ESCITALOPRAM SANDOZ 15 mg COMPRIMIDOS ORODISPERSÍVEIS

Como usar ESCITALOPRAM SANDOZ 15 mg COMPRIMIDOS ORODISPERSÍVEIS
Introdução
Prospecto: informação para o paciente
Escitalopram Sandoz 10 mg comprimidos bucodispersáveis EFG
Escitalopram Sandoz 15mg comprimidos bucodispersáveis EFG
Escitalopram Sandoz 20 mg comprimidos bucodispersáveis EFG
Leia todo o prospecto detenidamente antes de começar a tomar este medicamento, porque contém informações importantes para si.
- Conserva este prospecto, porque pode ter que voltar a lê-lo.
- Se tiver alguma dúvida, consulte o seu médico ou farmacêutico.
- Este medicamento foi-lhe prescrito apenas para si, e não deve dá-lo a outras pessoas, embora tenham os mesmos sintomas que si, porque pode prejudicá-las.
- Se experimentar efeitos adversos, consulte o seu médico ou farmacêutico, mesmo que se trate de efeitos adversos que não aparecem neste prospecto. Ver seção 4.
Conteúdo do prospecto
- O que é Escitalopram Sandoz e para que é utilizado.
- O que precisa saber antes de começar a tomar Escitalopram Sandoz.
- Como tomar Escitalopram Sandoz.
- Posíveis efeitos adversos.
- Conservação de Escitalopram Sandoz.
- Conteúdo do envase e informação adicional.
1. O que é Escitalopram Sandoz e para que é utilizado
Escitalopram Sandoz contém o princípio ativo escitalopram. Escitalopram pertence a um grupo de antidepressivos denominados inibidores seletivos da recaptação de serotonina (ISRSs). Estes medicamentos actuam sobre o sistema serotoninérgico no cérebro aumentando o nível de serotonina. As alterações do sistema serotoninérgico são consideradas um fator importante no desenvolvimento da depressão e doenças relacionadas.
Escitalopram Sandoz contém escitalopram e está indicado para o tratamento da depressão (episódios depressivos maiores) e transtornos de ansiedade (tais como transtorno de angústia com ou sem agorafobia, transtorno de ansiedade social, transtorno de ansiedade generalizada e transtorno obsessivo-compulsivo).
Podem passar um par de semanas antes de que comece a se sentir melhor. Continue tomando escitalopram embora leve um tempo em notar alguma melhoria.
Deve consultar um médico se piora ou se não melhora.
2. O que precisa saber antes de começar a tomar Escitalopram Sandoz
Não tome Escitalopram Sandoz
- se é alérgico a escitalopram ou a algum dos outros componentes deste medicamento (incluídos na seção 6),
- se toma outros medicamentos que pertencem ao grupo denominado inibidores da MAO, incluindo selegilina (utilizada para o tratamento da doença de Parkinson), moclobemida (utilizada para o tratamento da depressão) e linezolid (um antibiótico),
- se padece de nascimento ou sofreu um episódio de alteração da frequência cardíaca (detectado em um ECG, prova que avalia o funcionamento do coração),
- se está tomando medicamentos para problemas de ritmo cardíaco ou que possam afetar o ritmo cardíaco (ver seção 2 “Outros medicamentos e Escitalopram Sandoz”).
Advertências e precauções
Consulte o seu médico ou farmacêutico antes de começar a tomar Escitalopram Sandoz. Informe o seu médico se padece algum outro transtorno ou doença, porque o seu médico pode precisar tê-lo em conta. Em concreto, informe o seu médico:
- se padece epilepsia. O tratamento com Escitalopram Sandoz deve ser interrompido se se produzirem convulsões pela primeira vez, ou se observa um incremento na frequência das convulsões (ver também seção 4 “Posíveis efeitos adversos”),
- se tem alterações na função do fígado ou rins. Pode que o seu médico precise ajustar a sua dose,
- se padece diabetes. O tratamento com Escitalopram Sandoz pode alterar o controlo glicémico. Pode ser necessário um ajuste da dose de insulina e/ou hipoglicemiante oral,
- se tem diminuição dos níveis de sódio no sangue,
- se tende a desenvolver facilmente hemorragias ou cardenais, ou se está grávida (ver “Gravidez, lactação e fertilidade),
- se está recebendo tratamento eletroconvulsivo,
- se padece uma doença coronária,
- se padece ou padecia problemas de coração ou sofreu recentemente um ataque cardíaco,
- se o ritmo do seu coração em repouso é lento e/ou sabe que pode ter uma diminuição de sal como resultado de uma diarreia grave e prolongada e vómitos (estando doente) ou uso de diuréticos,
- se experimenta latidos do coração rápidos ou irregulares, desfalecimento, colapso ou tontura ao levantar-se, que pode indicar um funcionamento anómalo do ritmo do coração,
- se tem ou teve anteriormente problemas de olhos, como certos tipos de glaucoma (incremento da tensão no olho).
Tenha em conta
Alguns pacientes com doença maníaco-depressiva podem entrar em uma fase maníaca. Isso se caracteriza por um cambio de ideias pouco comum e rápido, alegria desproporcionada e uma atividade física excessiva. Se si o experimenta, contacte com o seu médico.
Sintomas como inquietude ou dificuldade para sentar-se ou estar de pé, podem ocorrer também durante as primeiras semanas de tratamento. Informe o seu médico imediatamente se experimenta estes sintomas.
Alguns medicamentos como escitalopram (também chamados ISRS/IRSN) podem causar sintomas de disfunção sexual (ver seção 4). Em alguns casos, estes sintomas persistem após a suspensão do tratamento.
Pensamentos suicidas e piora da depressão ou transtorno de ansiedade
Se se encontra deprimido e/ou sofre um transtorno de ansiedade, pode que em algumas ocasiões tenha pensamentos de se danificar a si mesmo ou de suicídio. Estes podem ir aumentando ao tomar antidepressivos pela primeira vez, posto que todos estes medicamentos requerem um tempo para começar a fazer efeito, geralmente ao redor de umas duas semanas, embora em alguns casos possa ser maior o tempo.
Pode ser mais propenso a ter este tipo de pensamentos:
- se previamente teve pensamentos de suicídio ou autolesão,
- se é um adulto jovem. Informação de ensaios clínicos demonstrou um aumento do risco de condutas suicidas em adultos menores de 25 anos com doenças psiquiátricas que foram tratados com um antidepressivo.
Se em qualquer momento tem pensamentos de autolesionar-se ou de suicídio, contacte com o seu médico ou dirija-se directamente a um hospital.
Pode ajudá-lo dizer a um parente ou um amigo próximoque está deprimido ou que tem um transtorno de ansiedade e pedir-lhe que leia este prospecto. Pode perguntar-lhes se pensam que a sua depressão ou transtorno de ansiedade piorou, ou se estão preocupados com as mudanças na sua atitude.
Crianças e adolescentes
Escitalopram Sandoz não deve ser utilizado normalmente no tratamento de crianças e adolescentes menores de 18 anos. Assim como, deve saber que em pacientes menores de 18 anos existe um maior risco de efeitos adversos como tentativas de suicídio, pensamentos de suicídio e hostilidade (predominantemente agressão, comportamento de confrontação e irritação) quando ingerem esta classe de medicamentos. Pese a isso, o seu médico pode prescrever Escitalopram Sandoz comprimidos bucodispersáveis a pacientes menores de 18 anos quando decidir o que é mais conveniente para o paciente. Se o seu médico prescreveu Escitalopram Sandoz comprimidos bucodispersáveis a um paciente menor de 18 anos e deseja discutir esta decisão, por favor, volte ao seu médico. Deve informar o seu médico se algum dos sintomas descritos anteriormente progride ou experimenta complicações quando pacientes menores de 18 anos estão tomando Escitalopram Sandoz comprimidos bucodispersáveis. Além disso, os efeitos a longo prazo em relação à segurança, no crescimento, maturidade e desenvolvimento cognitivo e comportamental de Escitalopram Sandoz comprimidos bucodispersáveis neste grupo de idade não foram demonstrados ainda.
Outros medicamentos e Escitalopram Sandoz
Comunique ao seu médico ou farmacêutico se está utilizando, utilizou recentemente ou pudesse ter que utilizar qualquer outro medicamento.
Informe ao seu médico se está tomando algum dos seguintes medicamentos:
- “Inibidores não seletivos da monoaminooxidase (IMAOs)”, que contenham fenelzina, iproniazida, isocarboxazida, nialamida e tranilcipromina como princípios ativos. Se tomou algum destes medicamentos tem que esperar 14 dias antes de começar a tomar Escitalopram Sandoz. Depois de terminar com Escitalopram Sandoz devem transcorrer 7 dias antes de tomar algum destes medicamentos.
- “Inibidores seletivos da MAO-A reversíveis”, que contenham moclobemida (para o tratamento da depressão).
- “Inibidores da MAO-B irreversíveis”, que contenham selegilina (para o tratamento da doença de Parkinson). Estes incrementam o risco de efeitos secundários.
- O antibiótico linezolid.
- Litio (para o tratamento do transtorno maníaco-depressivo) e triptófano.
- Imipramina e desipramina (ambos para o tratamento da depressão).
- Sumatriptano e medicamentos semelhantes (para o tratamento da enxaqueca) e tramadol (utilizado contra a dor intensa). Estes aumentam o risco de efeitos secundários.
- Buprenorfina(utilizada para a dor intensa) porque incrementa o risco de síndrome serotoninérgico, um síndrome potencialmente mortal.
- Cimetidina, lansoprazol e omeprazol (para o tratamento das úlceras de estômago), fluconazol (para o tratamento de infecções por fungos), fluvoxamina (antidepressivo) e ticlopidina (para reduzir o risco de acidente cerebro-vascular). Estes podem causar aumento das concentrações no sangue de escitalopram.
- Erva de São João (Hypericum perforatum) – planta medicinal utilizada para a depressão.
- Ácido acetilsalicílico e medicamentos anti-inflamatórios não esteroideos (medicamentos utilizados para aliviar a dor ou para reduzir o risco de trombose, também chamados anticoagulantes). Estes podem aumentar a tendência a hemorragias.
- Warfarina, dipiridamol e fenprocumona (medicamentos utilizados para fluidificar o sangue, também chamados anticoagulantes). O seu médico controlará provavelmente o tempo de coagulação do sangue no início e no final do tratamento com Escitalopram Sandoz, para comprovar que a dose de anticoagulante é ainda adequada.
- Mefloquina (para o tratamento da malária), bupropion (para o tratamento da depressão) e tramadol (para o tratamento da dor intensa) devido ao possível risco de diminuir o limiar de convulsões.
- Neurolépticos (medicamentos para o tratamento da esquizofrenia, psicose) e antidepressivos (antidepressivos tricíclicos e ISRSs) devido ao possível risco de diminuir o limiar de convulsões.
- Flecainida, propafenona e metoprolol (usados em doenças cardiovasculares), clomipramina e nortriptilina (antidepressivos) e risperidona, tioridazina e haloperidol (antipsicóticos). Pode ser que a dose de Escitalopram Sandoz precise ser ajustada.
- Medicamentos que diminuem os níveis de potássio ou magnésio no sangue porque isso incrementa o risco de sofrer alterações do ritmo do coração, que supõem um risco para a vida.
Não tome Escitalopram Sandoz se está tomando medicamentos para problemas do ritmo cardíaco ou que possam afetar o ritmo cardíaco, p. ex., antiarrítmicos Clase IA e III, antipsicóticos (p. ex., derivados de fenotiazina, pimozida, haloperidol), antidepressivos tricíclicos, alguns antimicrobianos (p. ex., esparfloxacino, moxifloxacino, eritromicina IV, pentamidina, tratamento antimalaria particularmente halofantrina), alguns antihistamínicos (astemizol, hidroxicina, mizolastina). Contacte com o seu médico para qualquer consulta adicional.
Toma de Escitalopram Sandoz com alimentos, bebidas e álcool
Escitalopram Sandoz comprimidos bucodispersáveis pode ser tomado com ou sem alimentos (ver seção 3 “Como tomar Escitalopram Sandoz”).
Como com muitos medicamentos, não se recomenda a combinação de Escitalopram Sandoz e álcool, embora não se espere que Escitalopram Sandoz interaja com álcool.
Gravidez, lactação e fertilidade
Se está grávida ou em período de lactação, acredita que possa estar grávida ou tem intenção de ficar grávida, consulte o seu médico ou farmacêutico antes de utilizar este medicamento. Não tome escitalopram se está grávida ou em período de lactação a menos que si e o seu médico tenham analisado os riscos e benefícios implicados.
Se toma escitalopram durante os últimos 3 meses da gravidez tem que ser consciente que se podem observar no bebê recém-nascido os seguintes efeitos: dificuldade respiratória, pele azulada, ataques, mudanças da temperatura corporal, dificuldades para se alimentar, vómitos, açúcar baixo no sangue, rigidez ou flacidez muscular, reflexos intensos, tremores, inquietude, irritabilidade, letargia, choro constante, sonolência e dificuldades para dormir. Se o seu bebê recém-nascido tem algum destes sintomas, contacte com o seu médico imediatamente.
Certifique-se de que a sua parteira e/ou médico sabem que está em tratamento com escitalopram.
Durante a gravidez, particularmente nos últimos 3 meses, medicamentos como escitalopram podem aumentar o risco de uma doença grave em recém-nascidos, denominada hipertensão pulmonar persistente neonatal (HPPN), na qual o bebê respira rapidamente e se põe azulado. Estes sintomas costumam começar durante as primeiras 24 horas após o nascimento. Se aparecem no seu bebê deve contactar com a sua parteira e/ou médico imediatamente.
Se escitalopram for utilizado durante a gravidez, nunca se deve interromper bruscamente.
Se toma escitalopram na etapa final da gravidez pode produzir um maior risco de sangramento vaginal abundante pouco após o parto, especialmente se tem antecedentes de alterações hemorrágicas. O seu médico ou parteira devem saber que si está tomando escitalopram para poder aconselhá-lo.
É de esperar que escitalopram seja excretado através do leite materno.
Citalopram, um medicamento semelhante a escitalopram, demonstrou reduzir a qualidade do esperma em modelos animais. Teoricamente, este efeito poderia afetar a fertilidade, mas até à data não se observou o seu impacto na fertilidade humana.
Condução e uso de máquinas
Não conduza nem utilize ferramentas ou máquinas até que saiba como lhe afeta o tratamento com escitalopram.
Escitalopram Sandoz contém lactosee sódio
Se o seu médico lhe indicou que padece uma intolerância a certos açúcares, consulte com ele antes de tomar este medicamento.
Este medicamento contém menos de 1 mmol de sódio (23 mg) por comprimido bucodispersável; isto é, essencialmente “exento de sódio”.
3. Como tomar Escitalopram Sandoz
Siga exatamente as instruções de administração deste medicamento indicadas pelo seu médico ou farmacêutico. Em caso de dúvida, consulte novamente o seu médico ou farmacêutico.
Os comprimidos bucodispersíveis de Escitalopram Sandoz devem ser tomados todos os dias como uma única dose diária. Pode tomar Escitalopram Sandoz com ou sem alimentos.
Os comprimidos bucodispersíveis de Escitalopram Sandoz quebram-se com facilidade, por isso deve manusear os comprimidos com cuidado. Não manuseie os comprimidos com as mãos húmidas, pois os comprimidos podem quebrar.
- Segure a tira de blister pelas bordas e separe um blister do resto da tira, rasgando suavemente ao longo da linha perfurada.
- Retire cuidadosamente a tampa traseira do blister.
- Coloque o comprimido na língua. O comprimido desintegrar-se-á rapidamente e pode ser engolido sem água.
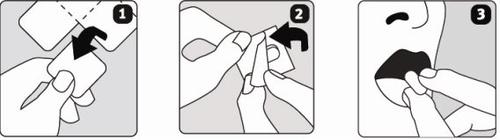
Escitalopram Sandoz comprimidos bucodispersíveis não está disponível para todas as doses que se descrevem abaixo. Para estas doses, deve tomar outros medicamentos disponíveis no mercado. Consulte o seu médico ou farmacêutico.
Adultos
Depressão
A dose recomendada de Escitalopram Sandoz é de 10 mg por dia, tomada como dose única. O seu médico pode aumentá-la até um máximo de 20 mg por dia.
Transtorno de ansiedade
A dose inicial de Escitalopram Sandoz é de 5 mg por dia, como dose única, durante a primeira semana, antes de aumentar a dose para 10 mg por dia. O seu médico pode aumentá-la posteriormente até um máximo de 20 mg por dia.
Transtorno de ansiedade social
A dose recomendada de Escitalopram Sandoz é de 10 mg por dia, tomada como dose única. O seu médico pode diminuir a dose para 5 mg por dia ou aumentá-la até um máximo de 20 mg por dia, dependendo de como responde ao medicamento.
Transtorno de ansiedade generalizada
A dose recomendada de Escitalopram Sandoz é de 10 mg por dia, tomada como dose única. A dose pode ser aumentada pelo seu médico até um máximo de 20 mg por dia.
Transtorno obsessivo-compulsivo
A dose recomendada de Escitalopram Sandoz é de 10 mg por dia, tomada como dose única. A dose pode ser aumentada pelo seu médico até um máximo de 20 mg por dia.
Pacientes idosos (maiores de 65 anos)
A dose inicial recomendada de Escitalopram Sandoz é de 5 mg por dia, tomada como dose única. A dose pode ser aumentada pelo seu médico até 10 mg por dia.
Uso em crianças e adolescentes
Escitalopram Sandoz normalmente não deve ser administrado a crianças e adolescentes. Para informações adicionais, ver seção 2 “O que precisa saber antes de começar a tomar Escitalopram Sandoz”.
Insuficiência renal
É aconselhada precaução em pacientes com função renal gravemente diminuída. Tome-o conforme o seu médico prescrever.
Insuficiência hepática
Os pacientes com problemas hepáticos não devem tomar mais de 10 mg por dia. Tome-o conforme o seu médico prescrever.
Pacientes considerados como metabolizadores lentos da CYP2C19
Os pacientes com este genótipo conhecido não devem tomar mais de 10 mg por dia. Tome-o conforme o seu médico prescrever.
Duração do tratamento
Podem passar um par de semanas antes de que comece a se sentir melhor. Continue tomando escitalopram mesmo que comece a se sentir melhor antes do previsto em sua condição.
Não modifique a dose do medicamento sem falar antes com o seu médico.
Continue tomando escitalopram pelo tempo recomendado pelo seu médico. Se interromper o tratamento demasiado cedo, os sintomas podem reaparecer. É recomendado que o tratamento continue durante pelo menos 6 meses após voltar a se sentir bem.
Se tomar mais Escitalopram Sandoz do que deve
Se tomar mais da dose prescrita de Escitalopram Sandoz, consulte imediatamente o seu médico, vá ao serviço de urgência do hospital mais próximo ou ligue para o Serviço de Informação Toxicológica, telefone: 91 562 04 20, indicando o medicamento e a quantidade ingerida. Faça-o mesmo quando não observe incômodos ou sinais de intoxicação. Alguns dos sinais de sobredose podem ser tontura, tremor, agitação, convulsão, coma, náuseas, vômitos, alterações no ritmo cardíaco, diminuição da pressão sanguínea e alterações no equilíbrio hidro/salino corporal. Leve o frasco de Escitalopram Sandoz se for ao médico ou ao hospital.
Se esquecer de tomar Escitalopram Sandoz
Não tome uma dose dupla para compensar as doses esquecidas. Se esquecer de tomar uma dose e se lembrar antes de ir para a cama, tome-a imediatamente. No dia seguinte, siga como sempre. Se se lembrar durante a noite ou no dia seguinte, deixe a dose esquecida e siga como sempre.
Se interromper o tratamento com Escitalopram Sandoz
Não interrompa o tratamento com Escitalopram Sandoz até que o seu médico o diga. Quando tiver terminado o seu curso de tratamento, geralmente é recomendado que a dose de Escitalopram Sandoz seja reduzida gradualmente durante várias semanas.
Quando deixar de tomar Escitalopram Sandoz, especialmente se for de forma brusca, pode sentir sintomas de abstinência. Estes são frequentes quando se suspende o tratamento com escitalopram. O risco é maior quando escitalopram é utilizado durante longo tempo, em doses elevadas ou quando a dose é reduzida muito rapidamente. A maioria das pessoas encontra que estes sintomas são leves e desaparecem por si mesmos em duas semanas. No entanto, em alguns pacientes podem ser intensos ou prolongados (2 a 3 meses ou mais). Se tiver sintomas graves de abstinência quando deixar de tomar escitalopram, contate o seu médico. Pode pedir-lhe que volte a tomar os comprimidos novamente e os deixe mais lentamente.
Os sintomas de abstinência incluem: sensação de tontura (inestável ou sem equilíbrio), sensação de formigamento, sensação de picada e (com menos frequência) de choque elétrico, mesmo na cabeça, alterações do sono (sonhos muito intensos, pesadelos, incapacidade de dormir), sensação de inquietude, dor de cabeça, sensação de tontura (náuseas), suoração (incluindo suores noturnos), sensação de inquietude ou agitação, tremor (inestabilidade), sentimento de confusão ou desorientação, sentimentos de emoção ou irritação, diarreia (fezes soltas), alterações visuais, pulsação rápida ou palpitações.
Se tiver alguma outra dúvida sobre o uso deste medicamento, pergunte ao seu médico ou farmacêutico.
4. Efeitos adversos possíveis
Assim como todos os medicamentos, este medicamento pode produzir efeitos adversos, embora nem todas as pessoas os sofram.
Os efeitos adversos normalmente desaparecem após algumas semanas de tratamento. Seja consciente de que muitos dos efeitos podem ser sintomas de sua doença e, portanto, melhorarão quando começar a se sentir melhor.
Se tiver algum dos seguintes sintomas, deve contatar o seu médico ou ir ao hospital imediatamente:
Pouco frequentes(podem afetar até 1 de cada 100 pessoas):
- sangramentos incomuns, incluindo sangramentos gastrointestinais.
Raros(podem afetar até 1 de cada 1.000 pessoas):
- inchaço da pele, língua, lábios ou face, ou tem dificuldades respiratórias ou de degluição (reação alérgica),
- febre alta, agitação, confusão, tremores e contrações repentinas de músculos, podem ser sinais de uma situação pouco comum denominada síndrome serotoninérgica (ver seção 2).
Frequência não conhecida(não pode ser estimada a partir dos dados disponíveis):
- dificuldades para urinar,
- convulsões (ataques), ver também a seção “Advertências e precauções”,
- pele amarelada e branqueamento nos olhos, são sinais de alteração da função hepática/hepatite,
- se experimenta batimentos cardíacos rápidos ou irregulares ou desfalecimento, sintomas que podem indicar uma condição de risco para a vida conhecida como Torsade de Pointes,
- pensamentos de se machucar (autolesão) ou pensamentos de suicídio, ver também a seção “Advertências e precauções”.
Além do indicado anteriormente, foram notificados os seguintes efeitos adversos:
Muito frequentes(podem afetar mais de 1 de cada 10 pessoas):
- sentir-se tonto (náuseas),
- dor de cabeça.
Frequentes(podem afetar até 1 de cada 10 pessoas):
- entupimento ou mucosidade nasal (sinusite),
- diminuição ou aumento do apetite,
- ansiedade, agitação, sonhos estranhos, dificuldade para conciliar o sono, sentir-se dormido, tontura, bocejos, tremores, coceira na pele,
- diarreia, constipação, vômitos, secura da boca,
- aumento da suoração,
- dores musculares e articulares (artralgia e mialgia),
- alterações sexuais (atraso da ejaculação, problemas com a ereção, diminuição da conduta sexual e as mulheres podem experimentar dificuldades para alcançar o orgasmo),
- fadiga, febre,
- aumento de peso.
Pouco frequentes(podem afetar até 1 de cada 100 pessoas):
- urticária, erupção cutânea, coceira (prurito),
- rangido de dentes, agitação, nervosismo, crise de ansiedade, confusão,
- alterações do sono, alterações do gosto, desmaios (síncope),
- dilatação da pupila (midriase), alteração visual, zumbidos nos ouvidos (tinnitus),
- perda de cabelo,
- hemorragia menstrual excessiva,
- período menstrual irregular,
- diminuição de peso,
- ritmo cardíaco rápido,
- inchaço de braços e pernas,
- hemorragia nasal.
Raros(podem afetar até 1 de cada 1.000 pessoas):
- agressividade, despersonalização, alucinações,
- ritmo cardíaco lento.
Frequência não conhecida(não pode ser estimada a partir dos dados disponíveis):
- diminuição dos níveis de sódio no sangue (os sintomas são sentir-se tonto e mal-estar com fraqueza muscular ou confusão),
- tontura ao se levantar devido à pressão sanguínea baixa (hipotensão ortostática),
- testes de função hepática alterados (aumento das enzimas hepáticas no sangue),
- transtornos do movimento (movimentos involuntários dos músculos),
- ereções dolorosas (priapismo),
- sinais de aumento do sangramento, p. ex., da pele ou mucosas (equimose),
- inchaço súbito da pele ou mucosas (angioedema),
- aumento da quantidade de urina excretada (secreção inadequada da hormona ADH),
- produção de leite em homens e em mulheres que não estão em período de amamentação,
- mania,
- foi observado um aumento do risco de fraturas ósseas em pacientes tratados com este tipo de medicamentos,
- alteração do ritmo cardíaco (denominada “prolongação do intervalo QT”, observada no ECG, atividade elétrica do coração),
- sangramento vaginal abundante pouco após o parto (hemorragia pós-parto), ver «Gravidez, lactação e fertilidade» na seção 2 para mais informações.
São conhecidos outros efeitos adversos que aparecem com medicamentos que atuam de forma semelhante ao escitalopram (princípio ativo de Escitalopram Sandoz), estes são:
- inquietude motora (acatisia),
- perda de apetite.
Comunicação de efeitos adversos
Se experimentar qualquer tipo de efeito adverso, consulte o seu médico ou farmacêutico, mesmo que se trate de possíveis efeitos adversos que não aparecem neste prospecto. Também pode comunicá-los diretamente através do Sistema Espanhol de Farmacovigilância de medicamentos de Uso Humano: https://www.notificaram.es. Mediante a comunicação de efeitos adversos, você pode contribuir para fornecer mais informações sobre a segurança deste medicamento.
5. Conservação de Escitalopram Sandoz
Mantenha este medicamento fora da vista e do alcance das crianças.
Não utilize este medicamento após a data de validade que aparece no frasco e blister após CAD/EXP. A data de validade é o último dia do mês que se indica.
Este medicamento não requer nenhuma temperatura especial de conservação.
Conservar no embalagem original para protegê-lo da luz e umidade.
Os medicamentos não devem ser jogados nos esgotos nem na lixeira. Deposite os frascos e os medicamentos que não precisa no Ponto SIGRE da farmácia. Em caso de dúvida, pergunte ao seu farmacêutico como se livrar dos frascos e dos medicamentos que não precisa. Dessa forma, ajudará a proteger o meio ambiente.
6. Conteúdo do frasco e informações adicionais
Composição de Escitalopram Sandoz
- O princípio ativo é escitalopram.
Escitalopram Sandoz 10 mg: cada comprimido bucodispersível contém 10 mg de escitalopram (como oxalato).
Escitalopram Sandoz 15 mg: cada comprimido bucodispersível contém 15 mg de escitalopram (como oxalato).
Escitalopram Sandoz 20 mg: cada comprimido bucodispersível contém 20 mg de escitalopram (como oxalato).
- Os demais componentes são: celulose microcristalina, lactose monohidrato, croscarmelosa sódica, polacrilina potássica, acesulfamo potássico, neohesperidina dihidrochalcona, estearato de magnésio, aroma de menta [contém: maltodextrina (de milho), amido modificado E 1450 (amido de milho ceroso) e óleo de menta (menta arvensis)], ácido clorídrico concentrado (para ajuste de pH).
Aspecto do produto e conteúdo do frasco
Escitalopram Sandoz 10 mg comprimidos bucodispersíveis: comprimidos de cor branca a branca opaca, redondos, planos com bordas biseladas, um diâmetro de 9 mm e gravado com "10" em um lado.
Escitalopram Sandoz 15 mg comprimidos bucodispersíveis: comprimidos de cor branca a branca opaca, redondos, planos com bordas biseladas, um diâmetro de 11 mm e gravado com "15" em um lado.
Escitalopram Sandoz 20 mg comprimidos bucodispersíveis: comprimidos de cor branca a branca opaca, redondos, planos com bordas biseladas, um diâmetro de 12 mm e gravado com "20" em um lado.
Escitalopram Sandoz comprimidos bucodispersíveis são envasados em blisters de papel descascável / PET / alumínio // PVC / alumínio / oPA, inseridos em um frasco de cartão.
Tamanhos de frasco:
7, 10, 12, 14, 20, 28, 30, 35, 50, 56, 60, 90, 98, 100 e 200 comprimidos bucodispersíveis.
Pode ser que apenas alguns tamanhos de frascos estejam comercializados.
Titular da autorização de comercialização e responsável pela fabricação
Titular da autorização de comercialização
Sandoz Farmacêutica, S.A.
Centro Empresarial Parque Norte
Edifício Carvalho
Rua Serrano Galvache, 56
28033 Madrid
Espanha
Responsável pela fabricação
Salutas Pharma GmbH
Otto-von-Guericke-Allee 1,
39179 Barleben
Alemanha
ou
Genepharm S.A.
18º km Marathon Avenue,
Pallini Attiki, 15351
Grécia
ou
LEK, S.A.
Ul Domaniewska 50 C
Varsóvia, PL 02-672
Polônia
ou
Lek Pharmaceuticals d.d.
Verovškova, 57
SL-1526 Ljubljana
Eslovênia
ou
S.C. Sandoz, S.R.L.
7A Livezeni Street, Targu-Mures
540472 Mures County, Romênia
ou
Rontis Hellas S.A. Medical and Pharmaceutical Products
Área Industrial de Larissa, P.O. Box 3012
GR41004 Larissa, Grécia
ou
Pharmadox Healthcare Ltd.
KW20A Kordin Industrial Park,
Paola, PLA 3000
Malta
Data da última revisão deste prospecto:11/2024
A informação detalhada deste medicamento está disponível na página web da Agência Espanhola de Medicamentos e Produtos Sanitários (AEMPS) http://www.aemps.gob.es/

Quanto custa o ESCITALOPRAM SANDOZ 15 mg COMPRIMIDOS ORODISPERSÍVEIS em Espanha em 2025?
O preço médio do ESCITALOPRAM SANDOZ 15 mg COMPRIMIDOS ORODISPERSÍVEIS em dezembro de 2025 é de cerca de 13.11 EUR. Os valores podem variar consoante a região, a farmácia e a necessidade de receita. Confirme sempre com uma farmácia local ou fonte online para obter informações atualizadas.
- País de registo
- Preço médio em farmácia13.11 EUR
- Substância ativa
- Requer receita médicaSim
- Fabricante
- Esta informação é apenas para referência e não constitui aconselhamento médico. Consulte sempre um médico antes de tomar qualquer medicamento. A Oladoctor não se responsabiliza por decisões médicas baseadas neste conteúdo.
- Alternativas a ESCITALOPRAM SANDOZ 15 mg COMPRIMIDOS ORODISPERSÍVEISForma farmacêutica: COMPRIMIDO, 10 mg escitalopramSubstância ativa: escitalopramFabricante: Lundbeck Espana S.A.Requer receita médicaForma farmacêutica: COMPRIMIDO, 15 mg escitalopramSubstância ativa: escitalopramFabricante: Lundbeck Espana S.A.Requer receita médicaForma farmacêutica: COMPRIMIDO, 20 mg escitalopramSubstância ativa: escitalopramFabricante: Lundbeck Espana S.A.Requer receita médica
Alternativas a ESCITALOPRAM SANDOZ 15 mg COMPRIMIDOS ORODISPERSÍVEIS noutros países
As melhores alternativas com o mesmo princípio ativo e efeito terapêutico.
Alternativa a ESCITALOPRAM SANDOZ 15 mg COMPRIMIDOS ORODISPERSÍVEIS em Polónia
Alternativa a ESCITALOPRAM SANDOZ 15 mg COMPRIMIDOS ORODISPERSÍVEIS em Ukraine
Médicos online para ESCITALOPRAM SANDOZ 15 mg COMPRIMIDOS ORODISPERSÍVEIS
Avaliação de posologia, efeitos secundários, interações, contraindicações e renovação da receita de ESCITALOPRAM SANDOZ 15 mg COMPRIMIDOS ORODISPERSÍVEIS – sujeita a avaliação médica e regras locais.









