ENBREL 10 mg PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL PARA USO PEDIÁTRICO


Como usar ENBREL 10 mg PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL PARA USO PEDIÁTRICO
Introdução
Prospecto: informação para o utilizador
Enbrel 10 mg pó e dissolvente para solução injetável para uso pediátrico
etanercept
Leia todo o prospecto atentamente antes de começar a usar este medicamento, porque contém informações importantes para si.
|
Conteúdo do prospecto
A informação que aparece neste prospecto é organizada nas seguintes 7 secções:
- O que é Enbrel e para que é utilizado
- O que precisa saber antes de começar a usar Enbrel
- Como usar Enbrel
- Posíveis efeitos adversos
- Conservação de Enbrel
- Conteúdo do envase e informação adicional
- Instruções de uso
1. O que é Enbrel e para que é utilizado
Enbrel é um medicamento que é fabricado a partir de duas proteínas humanas. Bloqueia a atividade de outra proteína, que se encontra no organismo, que produz inflamação. Enbrel actua reduzindo a inflamação associada a certas doenças.
Enbrel está indicado para o tratamento em crianças e adolescentes com as seguintes doenças:
- Para os seguintes tipos de artrite idiopática juvenil quando o tratamento com metotrexato não funcionou adequadamente, ou bem não é o adequado para a criança:
- Poliartrite (com fator reumatoide positivo ou negativo) e oligoartrite extendida em pacientes a partir de 2 anos.
- Artrite psoriásica em pacientes a partir de 12 anos.
- Para a artrite relacionada com entesite em pacientes a partir de 12 anos de idade para os quais o uso de outros tratamentos mais comumente utilizados não funcionou adequadamente, ou bem ditos tratamentos não são os adequados para eles.
- Psoríase grave em pacientes a partir de 6 anos de idade que tiveram uma resposta inadequada a (ou são incapazes de tomar) fototerapias ou outras terapias sistémicas.
2. O que precisa saber antes de começar a usar Enbrel
Não use Enbrel
- se a criança que está ao seu cuidado é alérgica a etanercept ou a algum dos outros componentes de Enbrel (incluídos na secção 6). Se a criança experimentar reações alérgicas, tais como opressão torácica, respiração ofegante, vertigem ou erupção, não injete mais Enbrel e entre em contato imediatamente com o seu médico.
- se a criança padece ou tem risco de desenvolver uma infecção grave do sangue, denominada sepsis. Se não tem certeza, consulte o seu médico.
- se a criança padece uma infecção de qualquer tipo. Se não tem certeza, consulte o seu médico.
Advertências e precauções
Consulte o seu médico antes de começar a usar Enbrel.
- Reações alérgicas:Se a criança experimentar reações alérgicas tais como opressão torácica, respiração ofegante, vertigem ou erupção, não injete mais Enbrel e entre em contato imediatamente com o seu médico.
- Látex:A tampa da seringa é feita de látex (borracha natural seca). Entre em contato com o seu médico antes de usar Enbrel se a seringa for manipulada por, ou se Enbrel for administrado a, alguém com hipersensibilidade (alergia) conhecida ou possível ao látex.
- Infecções/cirurgia:Se a criança desenvolver uma nova infecção ou estiver prestes a se submeter a uma intervenção cirúrgica maior, o médico pode ter interesse em controlar o tratamento da criança com Enbrel.
- Infecções/diabetes:Informe o seu médico se a criança tem histórico de infecções recorrentes ou se padece diabetes ou outros distúrbios que aumentem o risco de infecção.
- Infecções/monitorização:Informe o seu médico de qualquer viagem recente fora da região europeia. Se a criança desenvolver sintomas de uma infecção tais como febre, calafrios ou tosse, notifique-o imediatamente. O seu médico deve decidir se continuar a monitorizar a criança para verificar a presença de infecções após a criança deixar o tratamento com Enbrel.
- Tuberculose:Já que foram notificados casos de tuberculose em pacientes tratados com Enbrel, o seu médico examinará os sinais e sintomas de tuberculose antes de começar com Enbrel. Isso pode incluir um exame exhaustivo dos antecedentes médicos, radiografia torácica e uma prova de tuberculose. A realização desses exames deve ser registrada no Cartão de Informação do Paciente. É muito importante que diga ao seu médico se a criança teve tuberculose, ou se esteve em contato direto com alguém que teve tuberculose. Se os sintomas de tuberculose (tais como tosse persistente, perda de peso, apatia, febre moderada), ou alguma outra infecção aparecer durante ou após o tratamento, informe o seu médico imediatamente.
- Hepatite B:Informe o seu médico se a criança tem ou teve hepatite B alguma vez. O seu médico deve fazer a prova de hepatite B antes de a criança começar o tratamento com Enbrel. O tratamento com Enbrel pode reativar a hepatite B em pacientes que tenham estado previamente infectados pelo vírus da hepatite B. Se isso ocorrer, deve deixar de usar Enbrel.
- Hepatite C:Informe o seu médico se a criança tem hepatite C. O seu médico pode querer monitorizar o tratamento com Enbrel no caso de a infecção piorar.
- Distúrbios do sangue:Informe imediatamente o seu médico se a criança tem sintomas tais como febre persistente, dor de garganta, hematomas, sangramento ou palidez. Tais sintomas podem indicar a existência de um problema sanguíneo grave que faça necessária a interrupção do tratamento com Enbrel.
- Distúrbios do sistema nervoso e da visão:Informe o seu médico se a criança apresenta esclerose múltipla, neurite óptica (inflamação dos nervos ópticos) ou mielite transversa (inflamação da medula espinhal). O seu médico decidirá se Enbrel é um tratamento adequado.
- Insuficiência cardíaca congestiva:Informe o seu médico se a criança tem um histórico de insuficiência cardíaca congestiva, porque Enbrel precisa ser utilizado com precaução nessas circunstâncias.
- Câncer:Informe o seu médico se a criança tem ou teve linfoma (um tipo de câncer sanguíneo) ou qualquer outro câncer antes de a criança receber Enbrel.
Os pacientes com artrite reumatoide grave, que tiveram a doença durante muito tempo, podem correr um risco maior que o normal de desenvolver linfoma.
As crianças e adultos que estão tomando Enbrel podem ter um risco aumentado de desenvolver linfoma ou outro câncer.
Algumas crianças e adolescentes que receberam Enbrel ou outros medicamentos que funcionam da mesma maneira que Enbrel desenvolveram cânceres, incluindo tipos incomuns, que algumas vezes resultaram na morte.
Algumas crianças que receberam Enbrel desenvolveram cânceres de pele. Informe o seu médico se a criança desenvolver qualquer alteração na aparência da pele ou crescimentos na pele.
- Varicela:Informe o seu médico se a criança está exposta à varicela enquanto utiliza Enbrel. O seu médico determinará se é apropriado o tratamento preventivo para a varicela.
- Alcoolismo:Enbrel não deve ser usado para o tratamento de hepatite relacionada com alcoolismo. Por favor, informe o seu médico se a criança que está ao seu cuidado tem um histórico de alcoolismo.
- Granulomatose de Wegener:Não se recomenda Enbrel para o tratamento de granulomatose de Wegener, uma doença inflamatória rara. Se a criança que está ao seu cuidado tem granulomatose de Wegener, comente com o seu médico.
- Medicamentos antidiabéticos:Informe o seu médico se a criança tem diabetes ou está tomando medicamentos para tratar a diabetes. O seu médico pode decidir se a criança precisa de menos medicamento antidiabético enquanto toma Enbrel.
Crianças e adolescentes
Vacinações:Se possível, as crianças devem ter todas as vacinações atualizadas antes de utilizar Enbrel. Algumas vacinas, como a vacina da poliomielite oral, não devem ser administradas enquanto se está utilizando Enbrel. Consulte o médico da criança antes de a criança utilizar qualquer vacina.
Normalmente Enbrel não deve ser usado em crianças menores de 2 anos com poliartrite ou oligoartrite extendida, em crianças menores de 12 anos com artrite relacionada com entesite ou artrite psoriásica, nem em crianças menores de 6 anos com psoríase.
Outros medicamentos e Enbrel
Informe o seu médico ou farmacêutico se a criança está utilizando, utilizou recentemente ou pode ter que utilizar qualquer outro medicamento (incluindo anakinra, abatacept ou sulfassalazina), mesmo aqueles não prescritos pelo médico da criança. A criança não deve usar Enbrel junto com medicamentos que contenham os princípios ativos anakinra ou abatacept.
Gravidez e lactação
Enbrel só deve ser utilizado durante a gravidez se for claramente necessário. Consulte o seu médico se está grávida, acredita que possa estar grávida ou tem intenção de ficar grávida.
Se recebeu Enbrel durante a gravidez, o seu bebê pode apresentar um maior risco de contrair uma infecção. Além disso, em um estudo se observaram mais defeitos de nascimento quando a mãe havia recebido Enbrel durante a gravidez, em comparação com as mães que não haviam recebido Enbrel nem outros medicamentos semelhantes (antagonistas do TNF), mas não houve nenhum padrão nos tipos de defeitos de nascimento notificados. Outro estudo não encontrou um maior risco de defeitos congénitos quando a mãe havia recebido Enbrel durante a gravidez. O seu médico o ajudará a decidir se os benefícios do tratamento superam o risco potencial para o seu bebê.
Consulte o seu médico se deseja amamentar enquanto está em tratamento com Enbrel. É importante que informe o pediatra e outros profissionais de saúde sobre o uso de Enbrel durante a gravidez e a lactação antes de o seu bebê receber qualquer vacina.
Condução e uso de máquinas
Não se espera que o uso de Enbrel afete a sua capacidade para conduzir e usar máquinas.
3. Como usar Enbrel
Uso em crianças e adolescentes
Siga exatamente as instruções de administração deste medicamento indicadas pelo seu médico. Em caso de dúvida, consulte novamente o seu médico ou farmacêutico.
Se estima que a ação de Enbrel é demasiado forte ou fraca, comunique-o ao seu médico ou farmacêutico.
A dose adequada e a frequência de dosagem dependerão do peso corporal e da doença da criança ou adolescente. O médico indicará como agir para preparar e medir a dose adequada.
O frasco de 10 mg é para crianças a quem foi prescrito uma dose igual ou inferior a 10 mg. Cada frasco é para um único uso e para um único paciente, devendo ser descartada a solução sobrante que possa ficar no frasco.
Para poliartrite ou oligoartrite extendida em pacientes a partir de 2 anos de idade, ou artrite relacionada com entesite ou artrite psoriásica em pacientes a partir de 12 anos, a dose habitual é 0,4 mg de Enbrel por kg de peso corporal (até um máximo de 25 mg) duas vezes por semana, ou 0,8 mg de Enbrel por kg de peso corporal (até um máximo de 50 mg) uma vez por semana.
Para psoríase em pacientes a partir de 6 anos de idade, a dose habitual é 0,8 mg de Enbrel por kg de peso corporal (até um máximo de 50 mg) uma vez por semana. Se Enbrel não tiver efeito sobre a doença da criança após 12 semanas, o seu médico pode indicar que deixe de usar este medicamento.
Forma e via de administração
Enbrel é administrado mediante uma injeção sob a pele (mediante injeção subcutânea).
Enbrel pode ser administrado com ou sem alimentos ou bebidas.
O pó deve ser dissolvido antes do seu uso. Nasecção 7, “Instruções de uso”, são incluídas instruções detalhadas para a preparação e injeção de Enbrel.A solução de Enbrel não deve ser misturada com nenhum outro medicamento.
Para que o ajude a lembrar, pode ser útil anotar num diário quais dias da semana deve utilizar Enbrel.
Se usar mais Enbrel do que deve
Se você usar mais Enbrel do que devia (bem por injetar uma quantidade elevada em uma única ocasião ou bem por usá-lo com muita frequência), deve falar com um médico ou farmacêutico imediatamente. Leve sempre consigo o estojo do medicamento, mesmo que esteja vazio.
Se esquecer de injetar Enbrel
Se se esquecer de uma dose, deve injetá-la assim que você se lembrar, a não ser que a próxima dose esteja programada para o dia seguinte, caso em que deve omitir a dose esquecida. A seguir, continue injetando o medicamento no(s) dia(s) habitual(is). Se você não se lembrar até o dia em que deve ser administrada a próxima dose, não administre ao seu filho uma dose dupla (2 doses no mesmo dia) para compensar a dose esquecida.
Se interromper o tratamento com Enbrel
Os seus sintomas podem voltar após a interrupção do tratamento.
Se tiver alguma outra dúvida sobre o uso deste medicamento, pergunte ao seu médico ou farmacêutico.
4. Possíveis efeitos adversos
Tal como todos os medicamentos, este medicamento pode produzir efeitos adversos, embora nem todas as pessoas os sofram.
Reacções alérgicas
Se observar algum dos seguintes sintomas no criança, não administre mais Enbrel ao criança. Informe o seu médico imediatamente ou leve o criança ao Serviço de Urgências do hospital mais próximo.
- Dificuldade para engolir ou respirar.
- Inchaço do rosto, garganta, mãos e pés.
- Sensação de nervosismo ou ansiedade, palpitações, rubor súbito da pele e/ou sensação de calor.
- Erupção grave, picazón ou urticária (manchas proeminente da pele, avermelhadas ou pálidas, frequentemente acompanhadas de picazón).
As reacções alérgicas graves são raras. Se o criança apresentar algum dos sintomas acima, pode ser devido a uma reacção alérgica a Enbrel, pelo que deve procurar atendimento médico de urgência imediatamente.
Efeitos adversos graves
Se notar algum dos seguintes efeitos, o criança pode necessitar de atendimento médico de urgência.
- Sinais de infecções graves, tais como febre alta que pode estar acompanhada de tosse, falta de ar, arrepios, fraqueza, ou de uma zona dolorosa, sensível, avermelhada e com sensação de calor na pele ou articulações do criança.
- Sinais de distúrbios sanguíneos, tais como hemorragia, hematoma ou palidez.
- Sinais de distúrbios do sistema nervoso, tais como formigamento ou entorpecimento, alterações da visão, dor ocular ou aparecimento de fraqueza em um braço ou perna.
- Sinais de insuficiência cardíaca ou agravamento da insuficiência cardíaca, tais como fadiga ou falta de ar com a atividade, inchaço dos tornozelos, sensação de plenitude no pescoço ou no abdômen, falta de ar durante a noite ou tosse, cor azulada das unhas do criança ou ao redor dos lábios do criança.
- Sinais de câncer: o câncer pode afetar qualquer parte do corpo, incluindo a pele e o sangue, e os possíveis sinais dependerão do tipo e localização do câncer. Estes sinais podem ser, entre outros, perda de peso, febre, inchaço (com ou sem dor), tosse persistente, presença de nódulos ou espessamentos na pele.
- Sinais de reações autoimunes(em que se desenvolvem anticorpos que podem danificar tecidos normais do corpo) tais como dor, picazón, fraqueza e respiração, pensamento, sensação ou visão anormal.
- Sinais de lúpus ou síndrome tipo lúpustais como mudanças de peso, erupção persistente, febre, dor muscular ou articular ou cansaço.
- Sinais de inflamação dos vasos sanguíneostais como dor, febre, rubor ou calor da pele, ou picazón.
Estes efeitos adversos são raros ou pouco frequentes, mas são estados graves (alguns deles, em raras ocasiões, podem ser fatais). Se estes sinais ocorrerem, informe o seu médico imediatamente ou acuda ao Serviço de Urgências do hospital mais próximo.
A seguir, são listados os efeitos adversos conhecidos de Enbrel, agrupados por ordem decrescente de frequência:
- Muito frequentes(podem afetar mais de 1 em cada 10 pessoas):
Infecções (incluindo resfriado, sinusite, bronquite, infecções do trato urinário e infecções da pele); reacções no local de injeção (incluindo hemorragia, hematoma, rubor, picazón, dor e inchaço) (não ocorrem com tanta frequência após o primeiro mês de tratamento; alguns pacientes desenvolveram reação no local de injeção que haviam utilizado recentemente); e dor de cabeça.
- Frequentes(podem afetar até 1 em cada 10 pessoas):
Reacções alérgicas; febre; erupção; picazón; anticorpos dirigidos contra os tecidos normais (formação de autoanticorpos).
- Pouco frequentes(podem afetar até 1 em cada 100 pessoas):
Infecções graves (incluindo pneumonia, infecções não superficiais da pele, infecções das articulações, infecção do sangue e infecções generalizadas); agravamento da insuficiência cardíaca congestiva; baixo número de glóbulos vermelhos, baixo número de glóbulos brancos, baixo número de neutrófilos (um tipo de glóbulos brancos); baixo número de plaquetas; câncer de pele (excluindo melanoma); inchaço localizado da pele (angioedema); urticária (manchas proeminente da pele, avermelhadas ou pálidas, frequentemente acompanhadas de picazón); inflamação ocular, psoríase (nova ou agravamento); inflamação dos vasos sanguíneos afetando múltiplos órgãos; aumento das enzimas hepáticas nos exames de sangue (em pacientes que também recebem tratamento com metotrexato, o aumento das enzimas hepáticas é frequente); cãibras abdominais e dor, diarreia, perda de peso ou sangue nas fezes (sinais de problemas intestinais).
- Raros(podem afetar até 1 em cada 1.000 pessoas):
Reacções alérgicas graves (incluindo inchaço localizado grave da pele e respiração ofegante); linfoma (um tipo de câncer sanguíneo); leucemia (câncer que afeta o sangue e a medula óssea); melanoma (um tipo de câncer de pele); combinação de baixo número de glóbulos vermelhos, glóbulos brancos e plaquetas; distúrbios do sistema nervoso (com fraqueza muscular grave e sinais e sintomas semelhantes aos da esclerose múltipla ou inflamação dos nervos ópticos ou da medula espinal); tuberculose; insuficiência cardíaca congestiva de nova ocorrência; convulsões; lúpus ou síndrome tipo lúpus (os sintomas podem incluir erupção persistente, febre, dor articular e cansaço); erupção cutânea, que pode levar à formação grave de bolhas e à descamação da pele; reacções liquenoides (erupção cutânea pruriginosa vermelha-morada e/ou linhas grossas branco-acinzentadas nas mucosas); inflamação do fígado causada pelo sistema imunológico (hepatite autoimune; em pacientes que também recebem tratamento com metotrexato, a frequência é pouco frequente); distúrbio imunológico que pode afetar os pulmões, a pele e os gânglios linfáticos (sarcoidose); inflamação ou cicatrização dos pulmões (em pacientes que também recebem tratamento com metotrexato, a frequência de inflamação ou cicatrização dos pulmões é pouco frequente); dano aos pequenos filtros dentro dos rins, o que leva a uma função renal deficiente (glomerulonefrite).
- Muito raros(podem afetar até 1 em cada 10.000 pessoas):
Insuficiência da medula óssea para produzir células sanguíneas cruciais.
- Frequência não conhecida(não pode ser estimada a partir dos dados disponíveis):
Carcinoma de células de Merkel (um tipo de câncer de pele); sarcoma de Kaposi, um câncer pouco comum relacionado com a infecção pelo vírus do herpes humano 8. O sarcoma de Kaposi geralmente se manifesta com maior frequência como lesões cutâneas de cor púrpura; ativação excessiva de glóbulos brancos associada com a inflamação (síndrome de ativação de macrófagos); reativação de hepatite B (uma infecção do fígado); agravamento de uma doença chamada dermatomiosite (inflamação e fraqueza dos músculos acompanhada de erupção cutânea).
Outros efeitos adversos em crianças e adolescentes
Os efeitos adversos observados em crianças e adolescentes, bem como suas frequências, são semelhantes aos anteriormente descritos.
Comunicação de efeitos adversos
Se experimentar algum tipo de efeito adverso, consulte o seu médico ou farmacêutico, mesmo que se trate de possíveis efeitos adversos que não aparecem neste prospecto. Também pode comunicá-los diretamente através do Sistema Português de Farmacovigilância de Medicamentos de Uso Humano. Ao comunicar efeitos adversos, você pode contribuir para fornecer mais informações sobre a segurança deste medicamento.
5. Conservação de Enbrel
Mantenha este medicamento fora da vista e do alcance das crianças.
Não use este medicamento após a data de validade que aparece no invólucro após “EXP”. A data de validade é o último dia do mês que se indica.
Conservar na geladeira (2 °C - 8 °C). Não congelar.
Antes de preparar a solução de Enbrel, pode ser conservado fora da geladeira a uma temperatura máxima de 25 °C, e durante um único período de até 4 semanas; após o qual, o medicamento não pode ser refrigerado novamente. Enbrel deve ser descartado se não for usado nas 4 semanas seguintes à sua retirada da geladeira. É recomendável que anote a data em que Enbrel foi retirado da geladeira e a data a partir da qual Enbrel deve ser descartado (não superior a 4 semanas desde a retirada do invólucro da geladeira). Esta nova data não deve exceder a data de validade que aparece no invólucro.
Após preparar a solução de Enbrel, é recomendado o uso imediato. No entanto, a solução pode ser usada durante 6 horas se for armazenada a uma temperatura máxima de 25 °C.
Não utilize este medicamento se observar que a solução não é transparente ou contém partículas. A solução deve ser transparente, de incolora a cor amarela pálida ou marrom pálida, sem grumos, escamas ou partículas.
Os medicamentos não devem ser jogados nos esgotos nem na lixeira. Pergunte ao seu farmacêutico como se livrar dos invólucros e dos medicamentos que já não precisa. Dessa forma, ajudará a proteger o meio ambiente.
6. Conteúdo do frasco e informações adicionais
Composição de Enbrel
O princípio ativo de Enbrel é etanercepte. Cada frasco de Enbrel 10 mg de pó e dissolvente para solução injetável para uso pediátrico contém 10 mg de etanercepte. Uma vez reconstituído, a solução contém 10 mg/ml de etanercepte.
Os outros componentes são:
Pó: Manitol (E421), sacarose e trometamol.
Dissolvente: Água para preparações injetáveis.
Aspecto do produto e conteúdo do frasco
Enbrel 10 mg de pó e dissolvente para solução injetável para uso pediátrico é apresentado como um pó branco com dissolvente para solução injetável (pó para injetável). Cada frasco contém 4 frascos, 4 seringas pré-carregadas de água para preparações injetáveis, 4 agulhas, 4 adaptadores do frasco e 8 compressas com álcool.
Título do autorização de comercialização: Pfizer Europe MA EEIG Boulevard de la Plaine 17 1050 Bruxelas Bélgica | |
Responsável pela fabricação: Pfizer Manufacturing Belgium NV Rijksweg 12, 2870 Puurs-Sint-Amands Bélgica |
Podem solicitar mais informações sobre este medicamento dirigindo-se ao representante local do titular da autorização de comercialização:
Espanha
Pfizer, S.L.
Tel: +34 91 490 99 00
Data da última revisão deste prospecto:
A informação detalhada deste medicamento está disponível no site da Agência Europeia de Medicamentos: http://www.ema.europa.eu.
- Instruções de uso
Esta seção divide-se nos seguintes apartados:
- Introdução
- Preparar-se para a injeção
- Preparar a dose de Enbrel para a injeção
- Adicionar o dissolvente
- Retirar a solução de Enbrel do frasco
- Colocar a agulha na seringa
- Escolher um local de injeção
- Preparar o local de injeção e injetar a solução de Enbrel
- Eliminação dos materiais
- Introdução
As seguintes instruções explicam como preparar e injetar Enbrel. Leia atentamente as instruções e siga-as passo a passo. O médico da criança ou sua enfermeira(o) lhe ensinará a técnica apropriada para a administração de uma injeção e a quantidade que deve ser administrada à criança. Não tente administrar à criança uma injeção até que esteja seguro de que entendeu como deve preparar e administrar a injeção.
Esta injeção não deve ser misturada na mesma seringa ou frasco com nenhum outro medicamento. Ver a seção 5 para consultar as instruções sobre como conservar Enbrel.
- Preparar-se para a injeção
- Lave muito bem as mãos.
- Selecione uma superfície de trabalho plana, limpa e bem iluminada.
- A bandeja deve conter todos os elementos listados a seguir. (Se algum dos elementos citados não estiver na bandeja, não use a bandeja e consulte seu farmacêutico). Use apenas os elementos citados. NÃOuse nenhuma outra seringa.
1 Frasco de Enbrel
1 Seringa pré-carregada contendo um dissolvente transparente e incolor (água para preparações injetáveis)
1 Agulha
1 Adaptador do frasco
2 Compressas com álcool
- Examine a data de validade nas etiquetas do frasco e da seringa. Não devem ser usados após o mês e ano que figuram nelas.
- Preparar a dose de Enbrel para a injeção
- Retire o conteúdo da bandeja.
- Retire a cápsula de plástico do frasco de Enbrel (ver Figura 1). NÃOretire o tampão cinza ou o anel de alumínio que rodeia a parte superior do frasco.
Figura 1
- Use uma nova compressa com álcool para limpar o tampão cinza do frasco de Enbrel. Depois de limpar, não toque no tampão com as mãos e evite que ele toque alguma superfície.
- Coloque o frasco em posição vertical e para cima sobre uma superfície plana e limpa.
- Retire o papel que cobre o envase do adaptador do frasco.
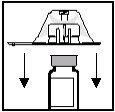
- Mantendo-o ainda no envase de plástico, coloque o adaptador do frasco sobre a parte superior do frasco de Enbrel, de forma que o punção do adaptador se encontre centrado dentro do círculo que aparece na parte superior do tampão do frasco (ver Figura 2).
Figura 2 | Figura 3 | Figura 4 |
|
|
|
CORRETO | INCORRETO |
- Mantenha o frasco firmemente com uma mão sobre a superfície plana. Com a outra mão, empurre firmemente o envase do adaptador EM LINHA RETAaté que perceba que o punção do adaptador penetra no tampão do frasco e ATÉ QUE PERCIBA E OUÇA QUE O BORDO DO ADAPTADOR SE ENCAIXAEM SEU LUGAR(ver Figura 3). NÃOempurre o adaptador formando um ângulo (ver Figura 4). É importante que o punção do adaptador do frasco penetre completamente no tampão do frasco.
- Enquanto mantém o frasco em uma mão, retire o envase de plástico do adaptador do frasco (ver Figura 5).
Figura 5
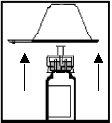
- Retire a cobertura protetora do extremo da seringa quebrando a cápsula branca ao longo da perfuração. Isso é realizado segurando o anel da cápsula branca enquanto se pega o extremo da cápsula branca com a outra mão e se dobra para cima e para baixo até que se quebre (ver Figura 6). NÃO RETIREo anel branco que permanece unido à seringa.
Figura 6
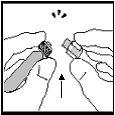
- Não use a seringa se a perfuração entre o extremo e o anel da cápsula estiver já quebrada. Comece de novo com outra bandeja de dose.
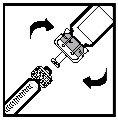
- Segurando o corpo de vidro da seringa (não o anel branco) em uma mão e o adaptador do frasco (não o frasco) na outra mão, conecte a seringa ao adaptador do frasco inserindo o extremo na abertura e girando no sentido dos ponteiros do relógio até que esteja completamente asegurada (ver Figura 7).
Figura 7
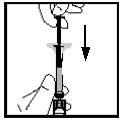
- Adicionar o dissolvente
- Enquanto se mantém o frasco em posição vertical sobre uma superfície plana, empurre o êmbolo MUITO LENTAMENTEaté que todo o dissolvente entre no frasco. Isso ajudará a reduzir a formação de espuma (muitas bolhas) (ver Figura 8).
- Uma vez que se adicionou o dissolvente a Enbrel, pode que o êmbolo se mova por si mesmo. Isso se deve à pressão de ar e não é motivo de preocupação.
Figura 8
- Com a seringa ainda inserida, mova lentamente o frasco em círculos algumas vezes, para dissolver o pó (ver Figura 9). NÃOagite o frasco. Espere até que todo o pó se tenha dissolvido (habitualmente menos de 10 minutos). A dissolução deve ser transparente e incolor ou de cor amarela pálida ou marrom pálida, sem grumos, escamas ou partículas. É normal que restem resíduos de espuma branca no frasco. NÃOuse Enbrel se não se dissolveu todo o pó do frasco em 10 minutos. Comece de novo com outra bandeja de dose.
Figura 9
- Retirar a solução de Enbrel do frasco
- O médico ou sua enfermeira(o) lhe terá indicado a quantidade adequada de solução que deve retirar do frasco. Se seu médico não lhe forneceu essas instruções, por favor, entre em contato com ele/ela.
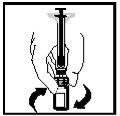
- Com a seringa ainda inserida no frasco e em seu adaptador, segure o frasco invertido ao nível dos olhos. Empurre o êmbolo completamente para o interior da seringa (ver Figura 10).
Figura 10
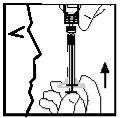
- Então, puxe lentamente o êmbolo para trás para retirar o líquido na seringa (ver Figura 11). Retire apenas a porção de líquido que o médico da criança lhe indicou. Depois de ter retirado Enbrel do frasco, pode encontrar um pouco de ar na seringa. Não se preocupe, pois o ar será eliminado em uma última etapa.
Figura 11
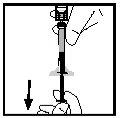
- Segure o frasco em posição invertida e separe a seringa do adaptador do frasco girando-a em sentido contrário ao dos ponteiros do relógio (ver Figura 12).
Figura 12
- Coloque a seringa cheia sobre uma superfície limpa e plana. Certifique-se de que o extremo não toque nada. Tenha cuidado para não apertar o êmbolo para baixo.
- Colocar a agulha na seringa
- A agulha está incluída em um envase de plástico para mantê-la estéril.
- Para abrir o envase de plástico, mantenha a parte mais curta e larga em uma mão. Coloque a outra mão sobre a parte mais longa do envase.
- Para quebrar o lacre, dobre o extremo maior para cima e para baixo até que se quebre (ver Figura 13).
Figura 13
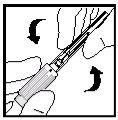
- Uma vez quebrado o lacre, retire a parte curta e larga do envase de plástico.
- A agulha permanecerá na parte mais longa do envase.
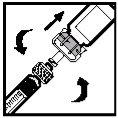
- Enquanto mantém a agulha e o envase em uma mão, pegue a seringa e insira seu extremo na abertura da agulha.
- Insira a seringa na agulha girando-a no sentido dos ponteiros do relógio até que esteja completamente asegurada (ver Figura 14).
Figura 14

- Retire o capuchão da agulha da seringa puxando firmemente, tendo cuidado para não tocar a agulha e evitando que a agulha toque alguma superfície (ver Figura 15). Tenha cuidado para não dobrar ou torcer o capuchão enquanto o retira para evitar danificar a agulha.
Figura 15

- Enquanto mantém a seringa em posição vertical, elimine as bolhas empurrando lentamente o êmbolo até extrair o ar (ver Figura 16).
Figura 16

- Escolher um local de injeção
- Os três locais recomendados para a injeção de Enbrel incluem: (1) a parte central da frente dos músculos da coxa; (2) o abdômen, exceto a área de 5 cm que rodeia o umbigo; e (3) a parte exterior superior dos braços (ver Figura 17). Se a criança se auto-injetar, não deve fazer isso na parte exterior superior dos braços.
Figura 17
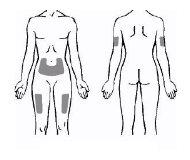
- Deve ser usado um local diferente para cada nova injeção. Cada nova injeção deve ser colocada, no mínimo, a 3 cm do local de injeção anterior. NÃOinjete em áreas de pele sensíveis, contundidas, avermelhadas ou endurecidas. Evite as áreas com cicatrizes ou estrias. (Pode ser útil anotar os locais de injeções anteriores).
- Se a criança tiver psoríase, tente não injetar diretamente em nenhuma zona de pele abultada, grossa, vermelha ou escamosa (“lesões psoriásicas da pele”).
- Preparar o local de injeção e injetar a solução de Enbrel
- Limpe o local de injeção em que vai injetar Enbrel com uma nova compressa impregnada em álcool, mediante um movimento circular. NÃOvolte a tocar nessa área até que a injeção seja administrada.
- Quando a área de pele limpa previamente se tiver secado, com uma mão, puxe a pele e segure-a firmemente. Com a outra mão, segure a seringa como um lápis.
- Com um movimento rápido e curto, empurre a agulha até o final, penetrando a pele com um ângulo entre 45º e 90º (ver Figura 18). Com a prática, encontrará o ângulo que é mais confortável para a criança. Tenha cuidado para não empurrar a agulha dentro da pele muito lentamente, ou com grande força.
Figura 18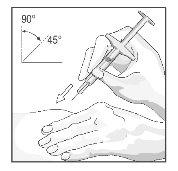
- Quando a agulha estiver completamente inserida dentro da pele, solte a pele que está segurando. Com a mão livre, segure a agulha perto de sua base para estabilizá-la. Depois, empurre o êmbolo para injetar toda a solução a uma velocidade lentae mantida (ver Figura 19).
Figura 19
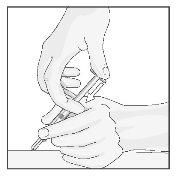
- Quando a seringa estiver vazia, retire a agulha da pele; tenha cuidado para manter a seringa no mesmo ângulo que estava quando foi inserida.
- Pressione com um algodão sobre o local de injeção durante 10 segundos. Pode ocorrer um ligeiro sangramento. NÃOfriccione o local de injeção. Se quiser, pode colocar um curativo ou bandagem.
- Eliminação dos materiais
- A seringa e a agulha NUNCAdevem ser reutilizadas. Elimine a agulha e a seringa seguindo as instruções de seu médico, enfermeira(o) ou farmacêutico.
Se tiver alguma dúvida, consulte um médico, enfermeira(o) ou farmacêutico que estejam familiarizados com o uso de Enbrel.
- País de registo
- Substância ativa
- Requer receita médicaSim
- Fabricante
- Esta informação é apenas para referência e não constitui aconselhamento médico. Consulte sempre um médico antes de tomar qualquer medicamento. A Oladoctor não se responsabiliza por decisões médicas baseadas neste conteúdo.
- Alternativas a ENBREL 10 mg PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL PARA USO PEDIÁTRICOForma farmacêutica: INJETÁVEL, 25 mgSubstância ativa: etanerceptFabricante: Samsung Bioepis Nl B.V.Requer receita médicaForma farmacêutica: INJETÁVEL, 50 mgSubstância ativa: etanerceptFabricante: Samsung Bioepis Nl B.V.Requer receita médicaForma farmacêutica: INJETÁVEL, 50mgSubstância ativa: etanerceptFabricante: Samsung Bioepis Nl B.V.Requer receita médica
Alternativas a ENBREL 10 mg PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL PARA USO PEDIÁTRICO noutros países
As melhores alternativas com o mesmo princípio ativo e efeito terapêutico.
Alternativa a ENBREL 10 mg PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL PARA USO PEDIÁTRICO em Ukraine
Médicos online para ENBREL 10 mg PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL PARA USO PEDIÁTRICO
Avaliação de posologia, efeitos secundários, interações, contraindicações e renovação da receita de ENBREL 10 mg PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL PARA USO PEDIÁTRICO – sujeita a avaliação médica e regras locais.