
COMIRNATY JN.1 30 microgramas/dose Dispersão Injetável

Pergunte a um médico sobre a prescrição de COMIRNATY JN.1 30 microgramas/dose Dispersão Injetável

Como usar COMIRNATY JN.1 30 microgramas/dose Dispersão Injetável
Introdução
Prospecto: informação para o utilizador
Comirnaty JN.1 30 microgramas/dose suspensão injetável
Adultos e adolescentes a partir de 12 anos
vacina de ARNm contra a COVID-19
bretovamerano
Este medicamento está sujeito a monitorização adicional, o que facilitará a detecção de nova informação sobre a sua segurança. Pode contribuir comunicando os efeitos adversos que o seu filho possa ter. A parte final da secção 4 inclui informação sobre como comunicar estes efeitos adversos.
Leia todo o prospecto atentamente antes de receber esta vacina, porque contém informação importante para si.
- Conserva este prospecto, porque pode ter que voltar a lê-lo.
- Se tiver alguma dúvida, consulte o médico do seu filho, farmacêutico ou enfermeiro.
- Se o seu filho experimentar efeitos adversos, consulte o médico do seu filho, farmacêutico ou enfermeiro, mesmo que se trate de efeitos adversos que não apareçam neste prospecto. Ver secção 4.
Conteúdo do prospecto
- O que é Comirnaty JN.1 e para que é utilizado
- O que precisa saber antes de o seu filho começar a receber Comirnaty JN.1
- Como é administrado Comirnaty JN.1
- Possíveis efeitos adversos
- Conservação de Comirnaty JN.1
- Conteúdo do envase e informação adicional
1. O que é Comirnaty JN.1 e para que é utilizado
Comirnaty JN.1 é uma vacina que é utilizada para prevenir a COVID-19 causada pelo SARS-CoV-2.
Comirnaty JN.1 30 microgramas/dose suspensão injetável é administrado a adultos e adolescentes a partir de 12 anos de idade.
A vacina faz com que o sistema imunológico (as defesas naturais do organismo) produza anticorpos e células sanguíneas que combatem o vírus, proporcionando assim proteção contra a COVID-19.
Como Comirnaty JN.1 não contém o vírus para produzir imunidade, não pode causar ao seu filho a COVID-19.
Esta vacina deve ser utilizada de acordo com as recomendações oficiais.
2. O que precisa saber antes de começar a receber Comirnaty JN.1
Comirnaty JN.1 não deve ser administrado
- se é alérgico ao princípio ativo ou a algum dos outros componentes deste medicamento (incluídos na secção 6).
Advertências e precauções
Consulte o seu médico, farmacêutico ou enfermeiro antes de receber a vacina se:
- teve alguma vez uma reação alérgica grave ou problemas para respirar após a injeção de qualquer outra vacina ou após ter recebido esta vacina no passado;
- está nervoso pelo processo de vacinação ou se desmaiou alguma vez após uma injeção com uma agulha;
- tem uma doença grave ou uma infecção com febre alta. No entanto, pode ser vacinado se tiver uma febre leve ou uma infecção das vias respiratórias altas como um resfriado;
- tem um problema hemorrágico, forma cardenais com facilidade ou usa um medicamento para prevenir a formação de coágulos de sangue;
- tem um sistema imunológico debilitado devido a uma doença como a infecção por VIH ou por algum medicamento, como os corticosteroides, que afetam o sistema imunológico.
Existe um maior risco de miocardite (inflamação do músculo cardíaco) e pericardite (inflamação do revestimento externo do coração) após a vacinação com Comirnaty (ver secção 4). Estes distúrbios podem aparecer a poucos dias da vacinação e foram produzidos principalmente num prazo de 14 dias. Foram observados com maior frequência após a segunda dose da vacinação, e com maior frequência em jovens do sexo masculino. O risco de miocardite e pericardite parece ser menor em crianças entre 5 e 11 anos de idade do que entre os 12 e os 17 anos de idade. A maioria dos casos de miocardite e pericardite se recuperou. Alguns casos requereram suporte de cuidados intensivos e foram observados casos mortais. Após a vacinação, deve estar alerta aos sinais de miocardite e pericardite, como dificuldade para respirar, palpitações e dor torácica, e deve procurar atenção médica imediata em caso de que apareçam.
Como com qualquer vacina, Comirnaty JN.1 pode não proteger completamente todas as pessoas que a recebam e não se sabe quanto tempo estará protegido.
A eficácia de Comirnaty JN.1 pode ser menor em pessoas imunocomprometidas. Se está imunocomprometido, é possível que receba doses adicionais de Comirnaty JN.1. Nesses casos, deve continuar a manter as precauções físicas para ajudar a prevenir a COVID-19.
Além disso, os seus contactos próximos devem ser vacinados conforme apropriado. Comente com o seu médico as recomendações individuais apropriadas.
Crianças
Não se recomenda utilizar Comirnaty JN.1 30 microgramas/dose suspensão injetável em crianças menores de 12 anos de idade.
Estão disponíveis formulações pediátricas para lactentes de 6 meses de idade e maiores e crianças menores de 12 anos de idade. Para mais informação, consulte o prospecto de outras formulações.
Não se recomenda utilizar a vacina em lactentes menores de 6 meses de idade.
Outros medicamentos e Comirnaty JN.1
Informa ao seu médico ou farmacêutico se está a utilizar, utilizou recentemente ou possa ter que utilizar qualquer outro medicamento ou recebeu recentemente qualquer outra vacina.
Comirnaty JN.1 pode ser administrado ao mesmo tempo que uma vacina antigripal.
Gravidez e amamentação
Se está grávida ou acha que pode estar grávida, informe o seu médico, enfermeiro ou farmacêutico antes de receber esta vacina.
Ainda não há dados disponíveis relativos ao uso de Comirnaty JN.1 durante a gravidez. No entanto, uma grande quantidade de informação sobre mulheres grávidas vacinadas com a vacina Comirnaty aprovada inicialmente durante o segundo e o terceiro trimestres não demonstrou efeitos negativos sobre a gravidez nem no recém-nascido. Embora a informação sobre os efeitos na gravidez ou no recém-nascido após a vacinação durante o primeiro trimestre seja limitada, não se observou qualquer alteração no risco de aborto espontâneo. Comirnaty JN.1 pode ser utilizado durante a gravidez.
Ainda não há dados disponíveis relativos ao uso de Comirnaty JN.1 durante a amamentação. No entanto, não se prevêem efeitos no recém-nascido/criança amamentada. Os dados sobre mulheres que estavam em período de amamentação após a vacinação com a vacina Comirnaty aprovada inicialmente não demonstraram um risco de efeitos adversos em crianças/recém-nascidos amamentados. Comirnaty JN.1 pode ser utilizado durante a amamentação.
Condução e uso de máquinas
Alguns dos efeitos da vacinação mencionados na secção 4 (Possíveis efeitos adversos) podem afetar temporariamente a capacidade para conduzir ou utilizar máquinas. Espere até que estes efeitos tenham desaparecido antes de conduzir ou utilizar máquinas.
3. Como é administrado Comirnaty JN.1
Comirnaty JN.1 é administrado sob a forma de injeção de 0,3 ml num músculo do braço.
Receberá 1 injeção, independentemente de ter recebido previamente uma vacina contra a COVID-19.
Se recebeu previamente uma vacina contra a COVID-19, não deve receber uma dose de Comirnaty JN.1 até pelo menos 3 meses após a dose mais recente.
Se está imunocomprometido, é possível que receba doses adicionais de Comirnaty JN.1.
Se tiver alguma outra dúvida sobre o uso de Comirnaty JN.1, pergunte ao seu médico, farmacêutico ou enfermeiro.
4. Possíveis efeitos adversos
Como todas as vacinas, Comirnaty JN.1 pode produzir efeitos adversos, embora nem todas as pessoas os sofram.
Efeitos adversos muito frequentes:podem afetar mais de 1 em cada 10 pessoas
- local de injeção: dor, inchaço
- fadiga, dor de cabeça
- dor muscular, dor nas articulações
- arrepios, febre
- diarreia
Alguns destes efeitos adversos foram ligeiramente mais frequentes em adolescentes entre 12 e 15 anos de idade do que em adultos.
Efeitos adversos frequentes:podem afetar até 1 em cada 10 pessoas
- náuseas
- vómitos («muito frequentes» em mulheres grávidas de 18 anos de idade e mais velhas e em pessoas imunocomprometidas entre 2 e 18 anos de idade)
- vermelhidão no local de injeção («muito frequente» em pessoas imunocomprometidas de 12 anos de idade e mais velhas)
- aumento do tamanho dos gânglios linfáticos (observado com maior frequência após uma dose de reforço)
Efeitos adversos pouco frequentes:podem afetar até 1 em cada 100 pessoas
- mal-estar, sensação de fraqueza ou falta de energia/sonolência
- dor no braço
- insónia
- coceira no local de injeção
- reações alérgicas, tais como erupção cutânea ou coceira
- diminuição do apetite
- tontura
- sudorese excessiva, sudorese noturna
Efeitos adversos raros:podem afetar até 1 em cada 1 000 pessoas
- paralisia temporária de um lado da face
- reações alérgicas, tais como urticária ou inchaço da face
Efeitos adversos muito raros:podem afetar até 1 em cada 10 000 pessoas
- inflamação do músculo cardíaco (miocardite) ou inflamação do revestimento externo do coração (pericardite) que pode dar lugar a dificuldade para respirar, palpitações ou dor torácica
Frequência não conhecida(não pode ser estimada a partir dos dados disponíveis)
- reação alérgica grave
- inchaço extenso no membro em que foi administrada a vacina
- inchaço da face (pode ocorrer inchaço da face em pacientes que tenham recebido injeções de preenchimento dérmico)
- uma reação cutânea que causa pontos vermelhos ou manchas na pele, que podem parecer um alvo ou um «olho de boi» com um centro de cor vermelha escura rodeado de anéis vermelhos mais pálidos (eritema multiforme)
- sensação anormal na pele, como formigamento ou picada (parestesia)
- diminuição da sensibilidade, especialmente na pele (hipoestesia)
- hemorragia menstrual abundante (a maioria dos casos não parecem ser graves e são de caráter temporário)
Comunicação de efeitos adversos
Se o seu filho experimentar qualquer tipo de efeito adverso, consulte o médico do seu filho, farmacêutico ou enfermeiro, mesmo que se trate de possíveis efeitos adversos que não apareçam neste prospecto.
Também pode comunicá-los diretamente através do sistema nacional de notificação incluído no Apêndice V.
Ao comunicar efeitos adversos, pode contribuir para fornecer mais informação sobre a segurança deste medicamento.
5. Conservação de Comirnaty JN.1
Mantenha este medicamento fora da vista e do alcance das crianças.
A seguinte informação sobre conservação, validade e uso e manipulação é destinada a profissionais de saúde.
Não utilize este medicamento após a data de validade que aparece na caixa e na etiqueta após CAD. A data de validade é o último dia do mês que se indica.
Conservar no congelador a uma temperatura entre –90 ºC e –60 ºC.
Conservar no embalagem original para protegê-lo da luz.
A vacina é recebida congelada a uma temperatura entre –90 °C e –60 °C. A vacina congelada pode ser conservada a uma temperatura entre –90 °C e –60 °C ou a uma temperatura entre 2 °C e 8 °C após a sua receção.
Viais monodose: Se conservados congelados a uma temperatura entre –90 °C e –60 °C, os envases de 10 viais monodose da vacina podem ser descongelados a uma temperatura entre 2 °C e 8 °C durante 2 horas ou podem ser descongelados viais individuais a temperatura ambiente (até 30 °C) durante 30 minutos
Viais multidose: Se conservados congelados a uma temperatura entre –90 °C e –60 °C, os envases de 10 viais da vacina podem ser descongelados a uma temperatura entre 2 °C e 8 °C durante 6 horas ou podem ser descongelados viais individuais a temperatura ambiente (até 30 °C) durante 30 minutos.
Viais descongelados (previamente congelados): Uma vez retirado do congelador, o vial não aberto pode ser conservado e transportado refrigerado a uma temperatura entre 2 °C e 8 °C durante um máximo de 10 semanas; não ultrapassar a data de validade impressa (CAD). O embalagem externo deve ser marcado com a nova data de validade a uma temperatura entre 2 °C e 8 °C. Uma vez descongelada, a vacina não pode ser recongelada.
Antes do seu uso, os viais não abertos podem ser conservados durante um máximo de 12 horas a temperaturas entre 8 °C e 30 °C.
Os viais descongelados podem ser manipulados em condições de luz ambiental.
Viais abertos: Após a primeira punção, conservar a vacina a uma temperatura entre 2 °C e 30 °C e utilizá-la num prazo de 12 horas, que inclui um tempo de transporte de até 6 horas. Eliminar a vacina não utilizada.
Não utilize esta vacina se observar partículas visíveis ou uma alteração de cor.
Os medicamentos não devem ser jogados nos esgotos nem na lixeira. Pergunte ao seu farmacêutico como se livrar dos envases e dos medicamentos que já não precisa. Dessa forma, ajudará a proteger o meio ambiente.
6. Conteúdo do frasco e informações adicionais
Composição de Comirnaty JN.1
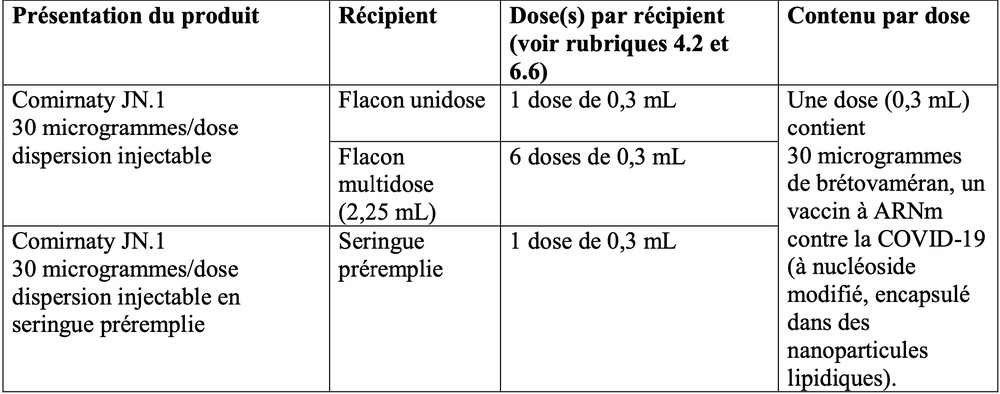
- O princípio ativo da vacina de ARNm contra COVID-19 (com nucleósidos modificados) é denominado bretovamerano.
- Um frasco monodose contém 1 dose de 0,3 ml com 30 microgramas de bretovamerano cada.
- Um frasco multidose contém 6 doses de 0,3 ml com 30 microgramas de bretovamerano cada.
- Os demais componentes são:
- ((4-hidroxibutil)azanodiil)bis(hexano-6,1-diil)bis(2-hexildecanoato) (ALC-0315)
- 2-[(polietilenglicol)-2000]-N,N-ditetradecilacetamida (ALC-0159)
- 1,2-diestearoil-sn-glicero-3-fosfocolina (DSPC)
- colesterol
- trometamol
- hidrocloruro de trometamol
- sacarose
- água para preparações injetáveis
Aspecto do produto e conteúdo do frasco
A vacina é uma dispersão (pH: 6,9-7,9) de cor entre branca e esbranquiçada que se apresenta em:
- um frasco monodose de 1 dose transparente (vidro de tipo I), de 2 ml, com um tampão de borracha e com uma cápsula de fechamento de plástico flip-off de cor cinza com um lacre de alumínio; ou
- um frasco multidose de 6 doses, transparente (vidro de tipo I), de 2 ml, com um tampão de borracha e com uma cápsula de fechamento de plástico flip-off de cor cinza com um lacre de alumínio.
Tamanho do frasco de frascos monodose: 10 frascos.
Tamanho do frasco de frascos multidose: 10 frascos.
Pode ser que apenas alguns tamanhos de frascos sejam comercializados
Título da autorização de comercialização
BioNTech Manufacturing GmbH
An der Goldgrube 12
55131 Mainz
Alemanha
Telefone: +49 6131 9084-0
Fax: +49 6131 9084-2121
Responsáveis pela fabricação
BioNTech Manufacturing GmbH
Kupferbergterrasse 17-19
55116 Mainz
Alemanha
Pfizer Manufacturing Belgium NV
Rijksweg 12
Puurs-Sint-Amands, 2870
Bélgica
Podem solicitar mais informações sobre este medicamento dirigindo-se ao representante local do titular da autorização de comercialização.
- Bélgica, Luxemburgo: Pfizer S.A./N.V.,
Tel: +32 (0)2 554 62 11
- República Tcheca: Pfizer, spol. s r.o., Tel: +420 283 004 111
- Dinamarca: Pfizer ApS, Tlf: +45 44 201 100
- Alemanha: BioNTech Manufacturing GmbH, Tel: +49 6131 90840
- Estônia: Pfizer Luxembourg SARL Eesti filiaal, Tel: +372 666 7500
- Grécia: Pfizer Ελλάς A.E., Τηλ.: +30 210 6785 800
- Espanha: Pfizer, S.L., Tel: +34914909900
- França: Pfizer, Tél +33 1 58 07 34 40
- Croácia: Pfizer Croatia d.o.o., Tel: +385 1 3908 777
- Irlanda: Pfizer Healthcare Ireland, Tel: 1800 633 363 (gratuito), +44 (0)1304 616161
- Islândia: Icepharma hf, Simi: +354 540 8000
- Itália: Pfizer S.r.l., Tel: +39 06 33 18 21
- Chipre: Pfizer Ελλάς Α.Ε. (Sucursal do Chipre), Tηλ: +357 22 817690
- Letônia: Pfizer Luxembourg SARL filiale Letônia, Tel.: +371 670 35 775
- Lituânia: Pfizer Luxembourg SARL filialas Lietuvoje, Tel. +370 52 51 4000
- Hungria: Pfizer Kft, Tel: +36 1 488 3700
- Malta: Vivian Corporation Ltd., Tel: +35621 344610
- Noruega: Pfizer AS, Tlf: +47 67 526 100
- Países Baixos: Pfizer BV, Tel: +31 (0)10 406 43 01
- Áustria: Pfizer Corporation Austria Ges.m.b.H, Tel: +43 (0)1 521 15-0
- Polônia: Pfizer Polska Sp. z o.o., Tel.: +48 22 335 61 00
- Portugal: Laboratórios Pfizer, Lda., Tel: +351 21 423 5500
- Romênia: Pfizer Romênia S.R.L, Tel: +40 (0) 21 207 28 00
- Eslovênia: Pfizer Luxembourg SARL, Pfizer, podružnica za svetovanje s podrocja farmacevtske dejavnosti, Liubliana, Tel.: +386 (0) 1 52 11 400
farmacêutica, Ljubljana, Tel.: +386 (0) 1 52 11 400
- República Eslovaca: Pfizer Luxembourg SARL, organizacná zložka, Tel: +421 2 3355 5500
- Finlândia: Pfizer Oy, Puh/Tel: +358 (0)9 430 040
- Suécia: Pfizer AB, Tel: +46 (0)8 550 520 00
- Reino Unido (Irlanda do Norte): Pfizer Limited, Tel: +44 (0) 1304 616161
Data da última revisão deste prospecto:
Escaneie o código com um dispositivo móvel para obter o prospecto em diferentes idiomas.

URL: www.comirnatyglobal.com
A informação detalhada deste medicamento está disponível no site da Agência Europeia de Medicamentos: https://www.ema.europa.eu.
-------------------------------------------------------------------------------------------------------------------------
Esta informação está destinada apenas a profissionais de saúde:
Administre Comirnaty JN.1 por via intramuscular como dose única de 0,3 ml independentemente da situação de vacinação prévia contra a COVID-19.
Para as pessoas que receberam previamente uma vacina contra a COVID-19, Comirnaty JN.1 deve ser administrado pelo menos 3 meses após a dose mais recente de uma vacina contra a COVID-19.
Podem ser administradas doses adicionais em pessoas que estejam gravemente imunocomprometidas.
Rastreabilidade
Com o objetivo de melhorar a rastreabilidade dos medicamentos biológicos, o nome e o número do lote do medicamento administrado devem estar claramente registrados.
Instruções para a manipulação antes do uso
Comirnaty JN.1 deve ser preparado por um profissional de saúde empregando uma técnica asséptica para garantir a esterilidade da dispersão preparada.
- Verifiqueque o frasco tem uma cápsula de plástico de cor cinzae que o nome do produto é Comirnaty JN.1 30 microgramas/dose dispersão injetável(pessoas de 12 anos de idade ou mais).
- Se o frasco tiver outro nome do produto na etiqueta, consulte a ficha técnica ou resumo das características do produto dessa formulação.
- Se o frasco for conservado congelado, deve ser descongelado antes do uso. Os frascos congelados devem ser transferidos para uma zona refrigerada entre 2 °C e 8 °C para descongelar. Certifique-se de que os frascos estão completamente descongelados antes de usá-los.
- Frascos monodose: um frasco de 10 frascos monodose pode levar 2 horas para descongelar.
- Frascos multidose: um frasco de 10 frascos multidose pode levar 6 horas para descongelar.
- Ao transferir os frascos para a conservação entre 2 °C e 8 °C, atualize a data de validade na caixa.
- Os frascos não abertos podem ser conservados por um máximo de 10 semanas a entre 2 °C e 8 °C; não ultrapasse a data de validade impressa (CAD).
- Como alternativa, os frascos congelados individuais podem ser descongelados durante 30 minutos a temperaturas de até 30 °C.
- Antes do uso, os frascos não abertos podem ser conservados por um máximo de 12 horas a temperaturas de até 30 °C. Os frascos descongelados podem ser manipulados em condições de luz ambiental.
Preparação de doses de 0,3 ml
- Misture suavemente os frascos invertendo-os dez vezes antes de usá-los. Não os agite.
- Antes de misturá-la, a dispersão descongelada pode conter partículas amorfas de cor entre branca e esbranquiçada.
- Depois de misturá-la, a vacina deve ter o aspecto de uma dispersão entre transparente e ligeiramente opalescente sem partículas visíveis. Não use a vacina se apresentar partículas visíveis ou uma mudança de cor.
- Verifique se o frasco é um frasco monodose ou multidose e siga as instruções para a manipulação aplicáveis que aparecem abaixo:
- Frascos monodose
- Extraia uma dose única de 0,3 ml de vacina.
- Elimine o frasco e o volume restante.
- Frascos multidose
- Os frascos multidose contêm 6 doses de 0,3 ml cada.
- Utilizando uma técnica asséptica, limpe o tampão do frasco com uma torunda antiséptica de um só uso.
- Extraia 0,3 ml de Comirnaty JN.1 para as crianças de 5 a 11 anos de idade.
Para extrair 6 doses de um mesmo frasco, devem ser usadas seringas e/ou agulhas com um volume morto baixo. A combinação de seringa e agulha com um volume morto baixo deve ter um volume morto de 35 microlitros como máximo. Se forem usadas seringas e agulhas convencionais, pode não haver volume suficiente para extrair uma sexta dose de um mesmo frasco.
- Cada dose deve conter 0,3 ml de vacina.
- Se a quantidade de vacina restante no frasco não puder proporcionar uma dose completa de 0,3 ml, elimine o frasco e o volume restante.
- Anote a hora e a data apropriadas no frasco. Elimine a vacina que não foi usada 12 horas após a primeira punção.
Eliminação
A eliminação do medicamento não utilizado e de todos os materiais que tenham estado em contato com ele será realizada de acordo com a regulamentação local.
- País de registo
- Substância ativa
- Requer receita médicaSim
- Fabricante
- Esta informação é apenas para referência e não constitui aconselhamento médico. Consulte sempre um médico antes de tomar qualquer medicamento. A Oladoctor não se responsabiliza por decisões médicas baseadas neste conteúdo.
- Alternativas a COMIRNATY JN.1 30 microgramas/dose Dispersão InjetávelForma farmacêutica: INJETÁVEL, 0.1 mg/mlSubstância ativa: covid-19, RNA-based vaccineFabricante: Biontech Manufacturing GmbhRequer receita médicaForma farmacêutica: INJETÁVEL, 0,2 mLSubstância ativa: covid-19, RNA-based vaccineFabricante: Biontech Manufacturing GmbhRequer receita médicaForma farmacêutica: INJETÁVEL, 0,3 microgramas/0,3 mlSubstância ativa: covid-19, RNA-based vaccineFabricante: Biontech Manufacturing GmbhRequer receita médica
Médicos online para COMIRNATY JN.1 30 microgramas/dose Dispersão Injetável
Avaliação de posologia, efeitos secundários, interações, contraindicações e renovação da receita de COMIRNATY JN.1 30 microgramas/dose Dispersão Injetável – sujeita a avaliação médica e regras locais.














