CLINIMIX N9G15E SOLUÇÃO PARA PERFUSÃO

Pergunte a um médico sobre a prescrição de CLINIMIX N9G15E SOLUÇÃO PARA PERFUSÃO

Como usar CLINIMIX N9G15E SOLUÇÃO PARA PERFUSÃO
Introdução
Prospecto: informação para o paciente
Clinimix N9G15E solução para perfusão
Leia todo o prospecto atentamente antes de iniciar a administração deste medicamento, porque contém informações importantes para si.
- Conserva este prospecto, porque pode ter que voltar a lê-lo.
- Se tiver alguma dúvida, consulte o seu médico ou enfermeiro.
- Se experimentar efeitos adversos, consulte o seu médico ou enfermeiro, mesmo que se trate de efeitos adversos que não aparecem neste prospecto. Ver secção 4.
Conteúdo do prospecto:
- O que é CLINIMIX e para que se administra
- O que necessita saber antes de iniciar a administração de CLINIMIX
- Como se administra CLINIMIX
- Possíveis efeitos adversos
- Conservação de CLINIMIX
- Conteúdo do envase e informação adicional
1. O que é Clinimix e para que se utiliza
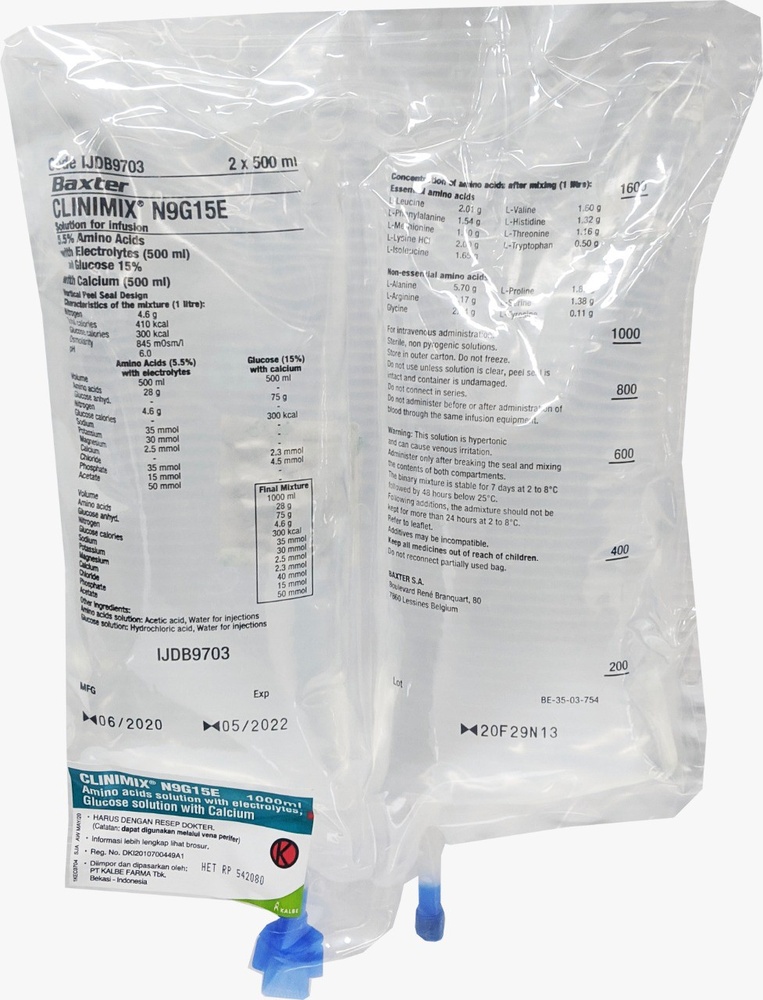
CLINIMIX é uma solução para perfusão. É fornecido em uma bolsa com 2 câmaras. Uma câmara contém uma solução de aminoácidos com eletrólitos e a segunda contém uma solução de glicose com cloreto de cálcio. As câmaras estão separadas por um selo não permanente. O conteúdo das câmaras deve ser misturado imediatamente antes da administração, enrolando a parte superior da bolsa para abrir os selos.
CLINIMIX é administrado para alimentar adultos e crianças através de um tubo conectado a uma veia quando a alimentação normal por via oral não é adequada.
CLINIMIX só deve ser administrado sob supervisão médica.
2. O que necessita saber antes de iniciar a administração de Clinimix
CLINIMIX não deve ser administrado se:
- é alérgico aos princípios ativos ou a qualquer um dos outros componentes deste medicamento (incluídos na secção 6),
- o seu organismo tem problemas para utilizar certos aminoácidos,
- tem demasiado açúcar no sangue (hiperglicemia grave),
- o seu sangue é excessivamente ácido (acidose metabólica devido a um excesso de lactato),
- tem um nível de sódio, potássio, magnésio, cálcio e/ou fósforo no sangue demasiado alto (hipernatremia, hiperpotassemia, hipermagnesemia, hipercalcemia e/ou hiperfosfatemia),
- em crianças com menos de 28 dias não se deve co-administrar ceftriaxona com soluções intravenosas que contenham cálcio, porque se podem formar partículas.
Em todos os casos, o seu médico decidirá se deve ser administrado este medicamento em função de factores como a idade, peso e estado clínico, juntamente com os resultados de todas as provas realizadas.
Advertências e precauções
Consulte o seu médico ou enfermeiro antes de que lhe administre CLINIMIX.
Se se desenvolver qualquer sinal anormal ou houver sintomas de uma reação alérgica, como febre, arrepios, erupções cutâneas ou dificuldade para respirar, suor excessivo, náuseas e dor de cabeça, diga-o ao seu médico ou enfermeiro: a perfusão será interrompida imediatamente. O seu médico supervisionará o seu estado enquanto lhe for administrado este medicamento e pode alterar a dose ou adicionar outros nutrientes, como lípidos, vitaminas, eletrólitos e oligoelementos se considerar adequado.
Certos medicamentos e doenças podem aumentar o risco de desenvolver uma infecção ou sepsis (bactérias no sangue). Há um risco especial de infecção ou sepsis quando um tubo (catéter intravenoso) é colocado na sua veia. O seu médico vigiará cuidadosamente para detectar qualquer sinal de infecção. O uso de técnicas assépticas (livres de germes) ao colocar e manter o catéter e ao fazer a fórmula nutricional pode reduzir o risco de infecção.
CLINIMIX com eletrólitos contém cálcio. Não deve ser administrado conjuntamente com o antibiótico ceftriaxona porque se podem formar partículas.
Se você está severamente desnutrido de tal forma que precisa receber alimentação através de uma veia, recomenda-se que a nutrição parenteral seja iniciada lentamente e com cuidado.
O seu médico supervisionará o seu estado no início da perfusão, especialmente se atualmente padece de problemas no fígado, rim, supra-renal, coração ou na circulação. O seu médico também deve ser consciente das condições graves que afetam a forma como o corpo lida com os açúcares, gorduras, proteínas ou sais (transtornos metabólicos). Se ocorrer qualquer sinal anormal, incluindo a irritação venosa, a perfusão deve ser interrompida.
Para verificar a eficácia e a segurança da administração, o seu médico realizará provas de laboratório e clínicas enquanto lhe for administrado este medicamento. Se lhe for administrado este medicamento durante várias semanas, o seu sangue será analisado regularmente. Em particular, em caso de intolerância à glicose, a glicose no sangue e na urina será controlada de forma rotineira, e, se você for um paciente diabético, a dose de insulina pode ter que ser adaptada.
Crianças e adolescentes
Quando for utilizado em recém-nascidos e crianças menores de 2 anos, a solução (nas bolsas e equipamentos de administração) deve ser protegida da exposição à luz até que a administração seja concluída. A exposição de Clinimix à luz ambiente, especialmente após misturas com oligoelementos e/ou vitaminas, gera peróxidos e outros produtos de degradação que podem ser reduzidos mediante a proteção da exposição à luz.
Interação de CLINIMIX com outros medicamentos
Informa o seu médico ou enfermeiro se está tomando ou tomou recentemente ou pode ter que tomar qualquer outro medicamento.
CLINIMIX com eletrólitos contém cálcio. Não deve ser administrado junto com o antibiótico ceftriaxona porque se podem formar partículas.
Devido ao conteúdo de potássio de CLINIMIX, deve-se prestar atenção especial com pacientes tratados com diuréticos poupadores de potássio (por exemplo, amilorida, espironolactona, triamtereno), inibidores do enzima conversor de angiotensina (ECA), antagonistas dos receptores de angiotensina II ou os imunossupressores tacrolimus ou ciclosporina, tendo em vista o risco de hiperpotassemia.
Gravidez, lactação e fertilidade
Se está grávida ou em período de lactação, ou acredita que possa estar grávida ou tem intenção de engravidar, consulte o seu médico antes de utilizar este medicamento.
3. Como se administra Clinimix
Antes de administrar o produto, deve-se romper o selo não permanente entre os dois compartimentos e misturar o conteúdo de ambos.
CLINIMIX pode ser administrado a adultos e crianças.
Trata-se de uma solução para perfusão que é administrada através de um tubo de plástico acoplado a uma veia do seu braço ou a uma veia grande do seu peito.
Quando for utilizado em recém-nascidos e crianças menores de 2 anos, a solução (nas bolsas e equipamentos de administração) deve ser protegida da exposição à luz até que a administração seja concluída (ver secção 2).
Dose – Adultos e crianças
O seu médico decidirá a dose que necessitará e o tempo durante o qual será administrado, em função da idade, peso e altura, estado clínico, volume diário de líquidos e necessidades de energia e nitrogênio.
Siga exatamente as instruções de administração de CLINIMIX indicadas pelo seu médico. Consulte o seu médico se tiver dúvidas.
A administração pode continuar durante tanto tempo quanto for necessário, em função do seu estado clínico.
A perfusão de uma bolsa geralmente dura entre 8 e 24 horas.
Se lhe administrarem mais CLINIMIX do que deve
Se a dose administrada for demasiado elevada ou a perfusão for demasiado rápida, pode fazer com que aumente o volume de circulação sanguínea ou que o sangue se torne excessivamente ácido. O conteúdo de glicose pode aumentar o nível de glicose do seu sangue e urina. A administração de um volume excessivo pode produzir náuseas, vómitos, tremores e alterações electrolíticas. Nesses casos, a perfusão deve ser interrompida imediatamente.
Em alguns casos graves, é possível que o seu médico tenha que submetê-lo a uma diálise renal temporária com o objetivo de ajudar os rins a eliminar o excesso de produto.
Para evitar que ocorram esses casos, o seu médico supervisionará regularmente o seu estado e analisará os seus parâmetros sanguíneos.
Se tiver alguma outra dúvida sobre o uso deste produto, pergunte ao seu médico.
4. Possíveis efeitos adversos
Como todos os medicamentos, este medicamento pode produzir efeitos adversos, embora nem todas as pessoas os sofram. Se observar algum cambio na forma de se sentir durante o tratamento ou após ele, comunique-o imediatamente ao seu médico ou enfermeiro.
As provas que o seu médico realizará enquanto lhe administrar este medicamento devem minimizar o risco de efeitos adversos.
A perfusão será interrompida imediatamente se se desenvolver qualquer sinal anormal ou sintomas de uma reação alérgica, como pressão arterial anormalmente alta ou baixa, a aparência de uma coloração azul ou púrpura da pele, frequência cardíaca anormalmente alta, dificuldade respiratória, vómitos, náuseas, erupções cutâneas, aumento da temperatura corporal, suor excessivo e arrepios.
Foram observados outros efeitos adversos, que se produzem com mais ou menos frequência:
- Anafilaxia (uma reação alérgica grave que é de início rápido e pode causar a morte).
- Níveis altos de glicose, amoníaco e compostos que contêm nitrogênio no sangue.
- Deterioração da função hepática, análise de sangue da função hepática anormal.
- Inflamação da vesícula biliar, presença de cálculos biliares na vesícula biliar.
- Inflamação das veias no local de infusão, irritação venosa, dor, irritação, ardor, tumefação.
- Presença de glicose na urina.
- Coma diabético
- Formação de partículas pequenas que bloqueiam os vasos sanguíneos pulmonares.
Comunicação de efeitos adversos:
Se experimentar qualquer tipo de efeito adverso, consulte o seu médico ou enfermeiro, mesmo que se trate de possíveis efeitos adversos que não aparecem neste prospecto. Também pode comunicá-los directamente através do Sistema Español de Farmacovigilância de medicamentos de Uso Humano: https://www.notificaram.es. Mediante a comunicação de efeitos adversos, você pode contribuir para proporcionar mais informações sobre a segurança deste medicamento.
5. Conservação de Clinimix
Mantenha este medicamento fora da vista e do alcance das crianças.
Quando for utilizado em recém-nascidos e crianças menores de 2 anos, a solução (nas bolsas e equipamentos de administração) deve ser protegida da exposição à luz até que a administração seja concluída (ver secção 2).
Não utilize este medicamento após a data de validade que aparece no envase e no embalagem exterior (MM/AAAA). A data de validade é o último dia do mês que se indica.
Não congele.
Conservar o envase no embalagem exterior.
Não jogue os medicamentos pelos desgotos nem na lixeira. Em caso de dúvida, pergunte ao seu farmacêutico como se livrar dos envases e dos medicamentos que não precisa. Dessa forma, ajudará a proteger o meio ambiente.
6. Conteúdo do envase e informação adicional
Composição de Clinimix
Os princípios ativos de cada bolsa da solução reconstituída são:
Princípios ativos | 1 l | 1,5 l | 2 l |
L-alanina | 5,70 g | 8,54 g | 11,39 g |
L-arginina | 3,17 g | 4,75 g | 6,33 g |
Glicina | 2,84 g | 4,25 g | 5,67 g |
L-histidina | 1,32 g | 1,98 g | 2,64 g |
L-isoleucina | 1,65 g | 2,48 g | 3,30 g |
L-leucina | 2,01 g | 3,02 g | 4,02 g |
L-lisina (como hidrocloruro de lisina) | 1,60 g (2,00 g) | 2,39 g (2,99 g) | 3,19 g (3,99 g) |
L-metionina | 1,10 g | 1,65 g | 2,20 g |
L-fenilalanina | 1,54 g | 2,31 g | 3,08 g |
L-prolina | 1,87 g | 2,81 g | 3,74 g |
L-serina | 1,38 g | 2,06 g | 2,75 g |
L-treonina | 1,16 g | 1,73 g | 2,31 g |
L-triptófano | 0,50 g | 0,74 g | 0,99 g |
L-tirosina | 0,11 g | 0,17 g | 0,22 g |
L-valina | 1,60 g | 2,39 g | 3,19 g |
Acetato de sódio 3H2O | 2,16 g | 3,23 g | 4,31 g |
Fosfato de potássio dibásico | 2,61 g | 3,92 g | 5,22 g |
Cloruro de sódio | 1,12 g | 1,68 g | 2,24 g |
Cloruro de magnésio 6H2O | 0,51 g | 0,77 g | 1,02 g |
Glucosa anidra (como glucosa monohidratada) | 75 g (83 g) | 113 g (124 g) | 150 g (165 g) |
Cloruro de cálcio 2H2O | 0,33 g | 0,50 g | 0,66 g |
Os demais componentes são:
- ácido acético, ácido clorídrico (para ajustar o pH da solução),
- água para preparações injetáveis.
Aspecto de CLINIMIX e conteúdo do envase
CLINIMIX é uma solução para perfusão que se apresenta em uma bolsa de plástico de várias camadas e com duas câmaras. O material da camada interior (de contato) da bolsa está fabricado de polímeros (mistura de copolímeros poliolefínicos) para ser compatível com os componentes e os aditivos autorizados. Outras camadas estão fabricadas de EVA (polietileno-vinil-acetato) e de um copoliéster.
Antes da reconstituição, as soluções de glucosa e aminoácidos são transparentes, incolores ou ligeiramente amareladas. Depois da reconstituição, a solução também é transparente, incolor ou ligeiramente amarelada.
Para evitar o contato com o oxigênio do ar, a bolsa está envasada no interior de uma sobrebolsa que atua como barreira de oxigênio, que contém um absorbente de oxigênio.
Tamanhos de envase
Bolsa de 1000 ml: caixa de cartão com 8 bolsas
1 bolsa de 1000 ml
Bolsa de 1500 ml: caixa de cartão com 6 bolsas
1 bolsa de 1500 ml
Bolsa de 2000 ml: caixa de cartão com 4 bolsas
1 bolsa de 2000 ml
Pode ser que apenas alguns tamanhos de envases estejam comercializados.
Título da autorização de comercialização
Baxter S.L.
Pouet de Camilo 2,
46394 Ribarroja del Turia (Valência) Espanha
Responsável pela fabricação
Baxter SA, Boulevard René Branquart, 80, 7860 Lessines, Bélgica
Este medicamento está autorizado nos estados membros do Espaço Económico Europeu com os seguintes nomes:
Clinimix N9G15E, solução para perfusão
Em alguns países está registado com outro nome, como se indica a seguir
Alemanha: Clinimix 3% GE
A última revisão deste prospecto foi em setembro de 2021
A informação detalhada deste medicamento está disponível na página web da Agência Espanhola de Medicamentos e Produtos Sanitários (AEMPS) http://www.aemps.gob.es/
-------------------------------------------------------------------------------------------------------------------
Esta informação está destinada unicamente a profissionais do sector sanitário:
- Composição quantitativa
Após misturar o conteúdo dos dois compartimentos, a composição da mistura binária para todos os tamanhos de bolsa disponíveis fornece o seguinte:
1 l | 1,5 l | 2 l | |
Azoto (g) Aminoácidos (g) Glucosa (g) | 4,6 28 75 | 6,8 41 113 | 9,1 55 150 |
Calorias totais (kcal) Calorias de glucosa (kcal) | 410 300 | 615 450 | 820 600 |
Sódio (mmol) Potássio (mmol) Magnésio (mmol) Cálcio (mmol) | 35 30 2,5 2,3 | 53 45 3,8 3,4 | 70 60 5,0 4,5 |
Acetato (mmol) Cloruro (mmol) Fosfato como HPO4 2- (mmol) | 50 40 15 | 75 60 23 | 100 80 30 |
pH Osmolaridade (mOsm/l) | 6 845 |
- Posologia e forma de administração.
Antes de administrar o produto, deve-se romper o selo não permanente entre os dois compartimentos e misturar o conteúdo de ambos.
Dose e velocidade de perfusão
A dose deve ser individualizada de acordo com as necessidades nutricionais/de líquidos do paciente, do gasto energético, estado clínico, peso corporal e da capacidade para metabolizar os componentes de Clinimix, bem como da energia ou proteínas adicionais administradas por via oral/enteral. Além disso, as necessidades diárias de líquidos, azoto e energia diminuem continuamente com a idade.
Em adultos, as necessidades oscilam entre 0,16 g de azoto/kg/dia (aproximadamente 1 g de aminoácido/kg/dia) e 0,32 g de azoto/kg/dia (aproximadamente 2 g de aminoácido/kg/dia).
Em lactentes, as necessidades oscilam entre 0,16 g de azoto/kg/dia (aproximadamente 1 g de aminoácido/kg/dia) e 0,40 g de azoto/kg/dia (aproximadamente 2,5 g de aminoácido/kg/dia).
Em adultos e pacientes de 12 a 18 anos as necessidades de calorias oscilam entre 25 kcal/kg/dia e 40 kcal/kg/dia, dependendo do estado de nutrição do paciente e do nível de catabolismo. Os pacientes menores de 12 anos podem ter requisitos mais altos.
Pode haver situações clínicas onde os pacientes requerem quantidades de nutrientes que diferem da composição de Clinimix. Nesta situação, qualquer ajuste de volume (dose) deve ter em conta o efeito resultante que terá sobre a dosagem de todos os demais componentes nutricionais de Clinimix. A velocidade e o volume da perfusão devem ser estabelecidos por um médico prescritor com experiência em fluidoterapia intravenosa pediátrica.
Este medicamento não contém os aminoácidos cisteína e taurina, considerados condicionalmente essenciais para neonatos e lactentes.
Este medicamento não se recomenda para recém-nascidos prematuros, a termo e para crianças menores de 2 anos.
A velocidade de administração deve ser ajustada em função da dose, das características da solução perfundida, da ingestão total de volume em 24 horas e da duração da perfusão.
O tempo de perfusão deve ser superior a 8 horas. Normalmente, a velocidade de administração aumenta gradualmente durante a primeira hora sem superar os 3 ml por quilograma de peso corporal por hora, e a dose máxima é 40 ml por quilograma de peso corporal ao dia.
Forma de administração
Quando se utilizar em recém-nascidos e crianças menores de 2 anos, a solução (nas bolsas e equipamentos de administração) deve ser protegida da exposição à luz até que finalize a administração.
Via de administração
Deve ser administrado por via intravenosa periférica ou central em função da osmolaridade final da mistura. Em geral, o limite aceito para a perfusão periférica é de aproximadamente 800 mOsm/l, mas varia consideravelmente em função da idade, do estado geral do paciente e das características das veias periféricas.
- Advertências e precauções especiais de emprego
ADVERTÊNCIAS
Com formulações de CLINIMIX foram notificadas reações de hipersensibilidade/à perfusão, incluindo hipotensão, hipertensão, cianose periférica, taquicardia, dispneia, vômitos, náuseas, urticária, erupção cutânea, prurido, eritema, hiperhidrose, febre e calafrios.
Com outros produtos de nutrição parenteral foi notificada anafilaxia.
Ao iniciar qualquer perfusão intravenosa é necessária uma monitorização clínica especial. Em caso de produzir-se sinal ou sintoma anormal, por exemplo uma reação de hipersensibilidade ou reação à perfusão, deve interromper-se a perfusão imediatamente.
As soluções que contêm glucosa devem ser usadas com precaução, em todo caso, em pacientes com alergia conhecida ao milho ou produtos derivados do milho.
Foram notificados precipitados vasculares pulmonares em pacientes que recebem nutrição parenteral.
Em alguns casos, foram produzidos resultados mortais. A adição excessiva de cálcio e fosfato aumenta o risco da formação de precipitados de fosfato de cálcio. Os precipitados foram notificados mesmo na ausência da sal de fosfato na solução. Também foram notificados precipitados distais no filtro em linha e se suspeita na formação de precipitado in vivo.
Se se apresentam sinais de sofrimento pulmonar, a perfusão deve ser interrompida e iniciada uma avaliação médica.
Além da inspeção da solução, o equipamento de infusão e o catéter também devem ser verificados periodicamente procurando precipitados.
Nos pacientes maiores de 28 dias (incluindo adultos), não deve ser administrado ceftriaxona por via intravenosa ao mesmo tempo que as soluções que contêm cálcio, incluindo CLINIMIX N9G15E, através da mesma via de perfusão. Se se utilizar a mesma linha de perfusão para a administração sequencial, deve limpar-se cuidadosamente com um líquido compatível entre as infusões.
A utilização de catéteres intravenosos para administrar formulações parenterais, um mau mantenimento dos catéteres ou as soluções contaminadas podem dar lugar a infecção e sepsis.
A imunossupressão e outros fatores, como a hiperglicemia, a desnutrição e/ou o estado de doença subjacente podem predispor os pacientes a complicações infecciosas.
O cuidado sintomático e o controle de laboratório de febre/calafrios, leucocitose, complicações técnicas com o dispositivo de acesso e a hiperglicemia podem ajudar a reconhecer as infecções precoces.
A aparição de complicações sépticas pode ser diminuída fazendo um maior ênfase no uso de uma técnica asséptica na colocação do catéter, no seu mantenimento, e na preparação da fórmula nutricional.
A realimentação de pacientes gravemente desnutridos pode dar lugar a um síndrome de realimentação, que se caracteriza pelo cambio do potássio, fósforo e magnésio intracelular dado que o paciente se encontra em estado anabólico. Também podem aparecer uma deficiência de tiamina e uma retenção de líquidos. A supervisão estrita e a ingestão gradual de nutrientes evitando a sobrealimentação podem prevenir estas complicações.
As soluções hipertônicas podem provocar irritação venosa se se perfundirem através de uma veia periférica. A escolha de uma veia periférica ou de uma veia central depende da osmolaridade final da mistura.
O limite aceito geralmente para uma perfusão periférica é ao redor de 800 mOsm/l, mas varia consideravelmente com a idade e estado geral do paciente e as características das veias periféricas.
Não conectar em série envases de plástico com o fim de evitar embolias gasosas devidas ao possível ar residual contido no envase primário.
PRECAUÇÕES
Antes de iniciar a perfusão, devem ser corrigidas as alterações graves no equilíbrio da água e os eletrólitos, os estados graves de sobrecarga de fluidos, e os transtornos metabólicos graves.
Podem produzir-se complicações metabólicas se a ingestão de nutrientes não se adaptar às necessidades do paciente, ou a capacidade metabólica de qualquer componente alimentício não for avaliada com precisão. Podem aparecer efeitos metabólicos adversos pela administração inadequada ou excessiva de nutrientes, ou pela composição de uma mistura não apropriada para as necessidades específicas do paciente.
É imprescindível realizar avaliações clínicas e determinações de laboratório com frequência para o correto controle durante a administração. Estas incluirão determinação de ionograma e provas funcionais do rim e do fígado.
Devem ser determinadas e controladas cuidadosamente as necessidades eletrólíticas dos pacientes que recebam estas soluções, sobretudo no caso de soluções sem eletrólitos.
A intolerância à glucosa é uma complicação metabólica comum em pacientes gravemente estressados. A perfusão desta solução pode produzir hiperglicemia, glicosúria e síndrome hiperosmolar. A glucosa no sangue e na urina deve ser controlada de forma rotineira e, se necessário, para os diabéticos deve adaptar-se a dose de insulina.
Usar com precaução em pacientes com insuficiência renal, especialmente se houver uma hiperpotasemia presente, devido ao risco de aparição ou piora da acidose metabólica e hiperazotemia se não se estiver realizando a eliminação extra-renal dos resíduos. O estado dos líquidos e eletrólitos deve ser controlado cuidadosamente nestes pacientes. Em caso de insuficiência renal grave, devem ser escolhidas soluções de aminoácidos especialmente formuladas.
Deve-se ter precaução ao administrar Clinimix aos pacientes com insuficiência suprarrenal.
Deve-se evitar a sobrecarga circulatória especialmente em pacientes com edema pulmonar, insuficiência e/ou falha cardíaca. O estado dos fluidos deve ser controlado cuidadosamente.
Além das provas da função hepática de rotina, nos pacientes com doença hepática preexistente ou insuficiência hepática devem ser controlados os possíveis sintomas de hiperamonemia.
É conhecido que em alguns pacientes com nutrição parenteral aparecem transtornos hepatobiliares incluyendo colestase, esteatose hepática, fibrose e cirrose, que podem dar lugar a insuficiência hepática, assim como colecistite e colelitíase. Acredita-se que a etiologia destes transtornos é multifatorial e pode diferir entre pacientes. Aqueles que desenvolvem parâmetros de laboratório anormais ou outros sinais de transtornos hepatobiliares devem ser avaliados rapidamente por um especialista clínico em doenças hepáticas com o fim de identificar os possíveis fatores causais e contribuintes, e as possíveis intervenções terapêuticas e profiláticas.
Nos pacientes que recebem soluções de aminoácidos pode ter lugar um aumento dos níveis de amônia no sangue e a hiperamonemia. Em alguns pacientes, isto pode indicar a presença de um transtorno congênito do metabolismo dos aminoácidos (ver a Seção 4. 3 da Ficha Técnica) ou de uma insuficiência hepática.
Deve-se medir com frequência o amoníaco no sangue em recém-nascidos e lactentes para detectar a hiperamonemia, que pode indicar a presença de uma anormalidade congênita do metabolismo dos aminoácidos.
De acordo com o grau e etiologia, a hiperamonemia pode requerer uma intervenção imediata.
Uma perfusão de aminoácidos demasiado rápida pode provocar náuseas, vômitos e calafrios. Nestes casos, há que interromper imediatamente a perfusão.
Em geral, a dose para os pacientes idosos deve ser cautelosa, tendo em conta a maior frequência de insuficiência hepática, renal ou cardíaca e de doenças concomitantes ou a farmacoterapia.
População pediátrica
- Não foram realizados estudos na população pediátrica.
- Ver mais acima em relação ao seguimento da hiperamonemia em pacientes pediátricos.
A exposição à luz das soluções para nutrição parenteral por via intravenosa, em especial após misturar-las com oligoelementos ou vitaminas, pode ter efeitos adversos no desenlace clínico dos recém-nascidos devido à geração de peróxidos e outros produtos de degradação. Quando se utilizar em recém-nascidos e crianças menores de 2 anos, Clinimix deve ser protegido da luz ambiental até que finalize a administração.
- Informação prática sobre a preparação e o manuseio
Precaução: Administre o produto unicamente após romper o selo e misturar o conteúdo dos dois compartimentos
1. |
| 2. |
| 3. |
|
Rompa desde a parte superior para abrir a sobrebolsa. | Retire a parte frontal da sobrebolsa para aceder à bolsa de Clinimix. Deseche a sobrebolsa e o sobrecito com o absorbente de oxigênio. | Coloque a bolsa sobre uma superfície horizontal e limpa com o asa à frente de você. | |||
4. |
| 5. |
| 6. |
|
Levante a zona do colgador para retirar a solução da parte superior da bolsa. Enrole firmemente a bolsa até que se abra completamente | Conecte o tubo de perfusão à bolsa. | Inicie a perfusão. | Verifique a perfusão. |
Misture o conteúdo invertendo a bolsa pelo menos 3 vezes.
Pendure a bolsa. Gire o protetor para retirá-lo do porto de administração. Conecte firmemente o conector de perfuração.
Use a solução apenas se for transparente, incolor ou ligeiramente amarelada, e se o envase não estiver danificado.
CLINIMIX deve estar à temperatura ambiente antes de seu uso.
A ativação de CLINIMIX pode ser realizada na sobrebolsa ou uma vez que esta tenha sido retirada.
Para uso único.
Não guarde os envases parcialmente utilizados e descarte todo o equipamento após utilizá-lo.
Não reconecte uma bolsa parcialmente utilizada.
Não conectar em série.
Quando for utilizado em recém-nascidos e crianças menores de 2 anos, deve proteger-se da exposição à luz até que finalize a administração. A exposição de Clinimix à luz ambiente, especialmente após misturá-lo com oligoelementos ou vitaminas, gera peróxidos e outros produtos de degradação que podem ser reduzidos se o produto for protegido da exposição à luz.
Suplementação
Devem ser fornecidos lípidos, vitaminas e oligoelementos aos pacientes que recebam alimentação parenteral durante um longo período de tempo.
Se for necessária a administração de aditivos, devem ser verificadas as compatibilidades e controlada a estabilidade das misturas.
A suplementação pode ser feita após abrir os selos não permanentes para todos os aditivos (uma vez que as duas soluções tenham sido misturadas). CLINIMIX pode ser suplementado com:
- Emulsões lipídicas (por exemplo, ClinOleic) a um ritmo de 50 a 250 ml por litro de CLINIMIX
CLINIMIX N9G15E 1 l + 100 ml de Lípidos 20%* | CLINIMIX N9G15E 1,5 l + 100 ml de Lípidos 20%* | CLINIMIX N9G15E 2 l + 250 ml de Lípidos 20%* | |
Azoto (g) Aminoácidos (g) Glicose (g) Lípidos (g) | 4,6 28 75 20 | 6,8 41 113 20 | 9,1 55 150 50 |
Calorias totais (kcal) Calorias de glicose (kcal) Calorias lipídicas (kcal) Relação glicose/lípidos | 610 300 200 60/40 | 815 450 200 69/31 | 1320 600 500 55/45 |
Sódio (mmol) Potássio (mmol) Magnésio (mmol) Cálcio (mmol) Acetato (mmol) Cloruro (mmol) Fosfato como HPO4 2- (mmol) | 35 30 2,5 2,3 50 40 15 | 53 45 3,8 3,4 75 60 23 | 70 60 5,0 4,5 100 80 30 |
pH Osmolaridade (mOsm/l) | 6 795 | 6 810 | 6 785 |
- Eletrólitos: por litro de CLINIMIX
Até uma concentração final de | Sódio | Potássio | Magnésio | Cálcio |
80 mmol | 60 mmol | 5,6 mmol | 3,0 mmol |
- Oligoelementos: por litro de CLINIMIX
Até uma concentração final de | Cobre | 10 μmol | Zinco | 77 μmol |
Cromo | 0,14 μmol | Manganês | 2,5 μmol | |
Flúor | 38 μmol | Cobalto | 0,0125 μmol | |
Selênio | 0,44 μmol | Molibdênio | 0,13 μmol | |
Iodo | 0,5 μmol | Ferro | 10 μmol |
- Vitaminas: por litro de CLINIMIX
Até uma concentração final de | Vitamina A | 1750 UI | Biotina | 35 μg |
Vitamina B6 | 2,27 mg | Vitamina B1 | 1,76 mg | |
Vitamina D | 110 UI | Ácido fólico | 207 μg | |
Vitamina B12 | 3,0 μg | Vitamina B2 | 2,07 mg | |
Vitamina E | 5,1 mg | Vitamina C | 63 mg | |
Vitamina PP | 23 mg | Vitamina B5 | 8,63 mg | |
Vitamina K | 75 μg |
Dados de estabilidade para a suplementação de CLINIMIX com outras emulsões lipídicas comercializadas e outros aditivos ou nutrientes estão disponíveis sob solicitação.
Se for observada a formação de uma leve creme, misture completamente a mistura por meio de uma suave agitação para obter uma emulsão uniforme antes da perfusão.
As adições devem ser feitas em condições assépticas.
As adições podem ser feitas com uma seringa ou com um equipamento de transferência.
- Adição com seringa ou equipamento de transferência com agulha.
- Prepare o porto de injeção (o tubo único, ver figura 1).
- Fure o tubo e injete.
- Misture a solução e os aditivos.
Incompatibilidades
Os aditivos podem ser incompatíveis, consulte o fabricante para obter mais detalhes.
Se for necessário adicionar aditivos, devem ser revisadas as compatibilidades e controlada a estabilidade das misturas.
A solução não deve ser administrada com, antes ou após uma administração de sangue através do mesmo equipamento, dada a possibilidade de pseudoaglutinação.
CLINIMIX N9G15E contém íons de cálcio, o que supõe um risco adicional de coagulação em sangue anticoagulado/conservado com citrato, ou seus componentes.
Como em qualquer mistura de nutrição parenteral, as proporções de cálcio e fosfato devem ser consideradas. A adição de um excesso de cálcio e fosfato, especialmente em forma de sais minerais, pode dar origem à formação de precipitados de fosfato de cálcio.
Como em outras soluções de perfusão que contêm cálcio, a administração concomitante de ceftriaxona e CLINIMIX N9G15E está contraindicada em recém-nascidos (≤ 28 dias de idade), embora se utilizem linhas de infusão separadas (risco de precipitação mortal da sal de cálcio com ceftriaxona no torrente sanguíneo do recém-nascido).
Em pacientes maiores de 28 dias (incluindo adultos), não deve ser administrada ceftriaxona por via intravenosa ao mesmo tempo que as soluções que contêm cálcio, incluindo as soluções de CLINIMIX N9G15E através da mesma via de perfusão (ver seção Advertências).
Se for utilizada a mesma linha de perfusão para a administração sequencial, deve ser limpa cuidadosamente com um líquido compatível entre as infusões.
- Período de validade
2 anos se conservado na sobrebolsa.
Recomenda-se utilizar o produto imediatamente após abrir o selo não permanente que há entre as 2 câmaras. No entanto, uma vez reconstituído (ou seja, após abrir o selo interno não permanente), demonstrou-se a estabilidade da solução reconstituída durante um máximo de 7 dias a uma temperatura entre 2 °C e 8 °C seguidos de um máximo de 48 h a uma temperatura que não supere os 25 °C.
Do ponto de vista microbiológico, as misturas devem ser utilizadas imediatamente após realizar as adições. Se não forem utilizadas imediatamente, o tempo de conservação em uso e as condições antes de sua utilização são responsabilidade do usuário, não devendo ser superior a 24 horas a 2-8ºC, a menos que as adições tenham sido realizadas em condições assépticas controladas e validadas. Se forem necessários períodos de conservação mais longos em condições excepcionais, pode-se contactar a empresa, pois dispõe de dados de estabilidade física e química em uso a 7 dias a 2-8ºC seguidos de 48 horas a temperatura inferior a 25ºC para os produtos indicados na seção anterior.
- País de registo
- Substância ativa
- Requer receita médicaSim
- Fabricante
- Esta informação é apenas para referência e não constitui aconselhamento médico. Consulte sempre um médico antes de tomar qualquer medicamento. A Oladoctor não se responsabiliza por decisões médicas baseadas neste conteúdo.
- Alternativas a CLINIMIX N9G15E SOLUÇÃO PARA PERFUSÃOForma farmacêutica: PERFURAÇÃO INJETÁVEL, 3,92 g / 1,26 g / 7,21 g / 3,36 g / 4,2 g / 5,11 g / 2,94 g / 2,8 g / 4,76 g / 5,07 g / 4,06 g / 14,49 g / 0,28 g / 8,05 g / 3,5 g / 200 gSubstância ativa: combinationsFabricante: Baxter S.L.Requer receita médicaForma farmacêutica: SOLUÇÃO INJETÁVEL PARA PERFUSÃO, 3,5 g / 200 g / 5,22 g / 1,88 g / 3,92 g / 1,26 g / 7,21 g / 3,36 g / 4,2 g / 5,11 g / 2,94 g / 2,8 g / 662 mg / 1,02 g / 4,76 g / 5,15 g / 5,07 g / 4,06 g / 14,49 g / 0,28 g / 8,05 gSubstância ativa: combinationsFabricante: Baxter S.L.Requer receita médicaForma farmacêutica: PERFURAÇÃO INJETÁVEL, 4,25 g / 300 g / 5,22 g / 1,54 g / 4,76 g / 1,53 g / 8,76 g / 4,08 g / 5,1 g / 6,2 g / 3,57 g / 3,4 g / 662 mg / 1,02 g / 5,78 g / 5,94 g / 6,16 g / 4,93 g / 17,6 g / 0,34 g / 9,78 gSubstância ativa: combinationsFabricante: Baxter S.L.Requer receita médica
Alternativas a CLINIMIX N9G15E SOLUÇÃO PARA PERFUSÃO noutros países
As melhores alternativas com o mesmo princípio ativo e efeito terapêutico.
Alternativa a CLINIMIX N9G15E SOLUÇÃO PARA PERFUSÃO em Polónia
Alternativa a CLINIMIX N9G15E SOLUÇÃO PARA PERFUSÃO em Ukraine
Médicos online para CLINIMIX N9G15E SOLUÇÃO PARA PERFUSÃO
Avaliação de posologia, efeitos secundários, interações, contraindicações e renovação da receita de CLINIMIX N9G15E SOLUÇÃO PARA PERFUSÃO – sujeita a avaliação médica e regras locais.