Como usar CIMZIA 200 mg SOLUÇÃO INJETÁVEL EM SERINGA PREENCHIDA
Introdução
Prospecto: informação para o utilizador
Cimzia 200 mg solução injetável em cartucho para dispensador de doses
certolizumab pegol
Leia todo o prospecto atentamente antes de começar a usar este medicamento, porque contém informações importantes para si.
- Conserva este prospecto, porque pode ter que voltar a lê-lo.
- Se tiver alguma dúvida, consulte o seu médico, farmacêutico ou enfermeiro.
- Este medicamento foi prescrito apenas para si, e não deve dá-lo a outras pessoas, mesmo que tenham os mesmos sintomas que si, porque pode prejudicá-las.
- Se experimentar efeitos adversos, consulte o seu médico, farmacêutico ou enfermeiro, mesmo que se trate de efeitos adversos que não aparecem neste prospecto. Ver secção 4.
Conteúdo do prospecto
- O que é Cimzia e para que é utilizado
- O que precisa saber antes de começar a usar Cimzia
- Como usar Cimzia
- Posíveis efeitos adversos
- Conservação de Cimzia
- Conteúdo do envase e informações adicionais
O seu médico também lhe dará uma “Tarjeta de Informação para o Paciente”, que contém informações de segurança importantes que deve conhecer antes de ser administrado Cimzia e enquanto durar o tratamento com este medicamento. Leve esta “Tarjeta de Informação para o Paciente” consigo.
1. O que é Cimzia e para que é utilizado
Cimzia contém o princípio ativo certolizumab pegol, um fragmento de anticorpo humano. Os anticorpos são proteínas que reconhecem e se unem especificamente a outras proteínas. Cimzia une-se a uma proteína específica chamada fator de necrose tumoral α (TNFα). Deste modo, este TNFα é bloqueado por Cimzia, o que diminui a inflamação em doenças como artrite reumatoide, espondiloartrite axial, artrite psoriásica e psoríase. Os medicamentos que se unem ao TNFα também são chamados bloqueantes do TNF.
Cimzia é utilizado em adultos para o tratamento das seguintes doenças inflamatórias:
- artrite reumatoide,
- espondiloartrite axial(incluindo espondilite anquilosante e espondiloartrite axial sem evidência radiográfica de espondilite anquilosante),
- artrite psoriásica
- psoríase em placas.
Artrite reumatoide
Cimzia é utilizado para tratar a artrite reumatoide. A artrite reumatoide é uma doença inflamatória das articulações. Se tem artrite reumatoide ativa de moderada a grave, primeiro pode receber outros medicamentos, normalmente metotrexato. Se não responder bem a estes medicamentos, será administrado Cimzia em combinação com metotrexato para tratar a artrite reumatoide. Se o seu médico determinar que o metotrexato é inapropriado, Cimzia pode ser administrado apenas.
Cimzia em combinação com metotrexato pode ser utilizado também para tratar a artrite reumatoide ativa, grave e progressiva sem usar previamente metotrexato ou outros tratamentos com medicamentos.
Cimzia será administrado em combinação com metotrexato, é utilizado para:
- reduzir os sinais e sintomas da doença,
- diminuir o dano no cartilagem e no osso das articulações causado pela doença,
- melhorar a função física e desenvolvimento das tarefas diárias.
Espondilite anquilosante e espondiloartrite axial sem evidência radiográfica de espondilite anquilosante.
Cimzia é utilizado para o tratamento da espondilite anquilosante e espondiloartrite axial sem evidência radiográfica de espondilite anquilosante (algumas vezes referida como espondiloartrite axial não radiográfica). Estas doenças são doenças inflamatórias da coluna. Se tem espondilite anquilosante ou espondiloartrite axial não radiográfica, primeiro será tratado com outros medicamentos. Se não responder bem a estes medicamentos, será administrado Cimzia para:
- reduzir os sinais e sintomas da doença,
- melhorar a função física e desenvolvimento das tarefas diárias.
Artrite psoriásica
Cimzia é utilizado para o tratamento da artrite psoriásica ativa. A artrite psoriásica é uma doença inflamatória das articulações, acompanhada normalmente por psoríase. Se tem artrite psoriásica ativa, primeiro será administrado outros medicamentos, normalmente metotrexato. Se não responder bem a estes medicamentos, será administrado Cimzia em combinação com metotrexato para:
- reduzir os sinais e sintomas da doença,
- melhorar a função física e desenvolvimento das tarefas diárias.
Se o seu médico determinar que o metotrexato não é o tratamento apropriado, Cimzia pode ser administrado apenas.
Psoríase em placas
Cimzia é utilizado para o tratamento da psoríase em placas de moderada a grave. A psoríase em placas é uma doença inflamatória da pele, que pode afetar também o couro cabeludo e as unhas.
Cimzia é utilizado para reduzir a inflamação da pele e outros sinais e sintomas da doença.
2. O que precisa saber antes de começar a usar Cimzia
NÃO use Cimzia
- Se é ALÉRGICO(hipersensível) a certolizumab pegol ou a qualquer um dos outros componentes deste medicamento (incluídos na secção 6).
- Se padece uma infecção grave, incluindo TUBERCULOSEativa (TB).
- Se padece INSUFICIÊNCIA CARDÍACAde moderada a grave. Informe o seu médico se teve ou tem algum problema cardíaco grave.
Advertências e precauções
Antes de iniciar o tratamento com Cimzia, informe o seu médico se:
Reações alérgicas
- Se experimenta REAÇÕES ALÉRGICAStais como opressão no peito, dificuldade para respirar, tontura, inchaço ou erupção, deixe de utilizar Cimzia e contacte IMEDIATAMENTEcom o seu médico. Algumas dessas reações poderiam ocorrer após a primeira administração de Cimzia.
- Se alguma vez teve uma reação alérgica ao látex.
Infecções
- Se teve INFECÇÕES RECORRENTESou OPORTUNÍSTICASou outras doenças que aumentam o risco de infecções (como o tratamento com imunossupressores, que são medicamentos que podem reduzir a sua capacidade para lutar contra as infecções).
- Se padece qualquer infecção ou se desenvolve sintomas como febre, feridas, cansaço ou problemas dentários. Enquanto estiver em tratamento com Cimzia, pode contrair uma infecção com mais facilidade, incluindo infecções graves, ou em casos raros infecções que poderiam por em perigo a sua vida.
- Foram descritos casos de TUBERCULOSE (TB)em pacientes em tratamento com Cimzia, o seu médico o examinará em busca de sinais ou sintomas de tuberculose antes de começar o tratamento com Cimzia. Isso incluirá a elaboração de um histórico médico minucioso, radiografia de tórax e a prova da tuberculina. A realização destas provas deve ser anotada na sua “Tarjeta de Informação para o Paciente”. Se for diagnosticada tuberculose latente (inativa), pode ser necessário que receba a medicação apropriada contra a tuberculose antes de iniciar o tratamento com Cimzia. Em raros casos, pode desenvolver-se tuberculose durante o tratamento, mesmo que tenha recebido um tratamento preventivo para a tuberculose. É muito importante que informe o seu médico em caso de ter padecido tuberculose ou ter estado em contacto com um paciente com tuberculose. Se aparecerem sintomas de tuberculose (tos persistente, perda de peso, mal-estar geral, febrícula) ou de qualquer outra infecção durante ou uma vez finalizado o tratamento com Cimzia, ponha-se em contacto imediatamente com o seu médico.
- Se está em perigo de contrair uma infecção por o VÍRUS DA HEPATITE B (VHB), se é portador do VHB ou se tem uma infecção ativa com VHB, Cimzia pode aumentar o risco de reativação do VHB em pessoas portadoras deste vírus. Se isso ocorrer, deve deixar de utilizar Cimzia. O seu médico deve fazer-lhe provas de VHB antes de começar o tratamento com Cimzia.
Insuficiência cardíaca
- Se padece INSUFICIÊNCIA CARDÍACAleve e está em tratamento com Cimzia, o seu médico deve fazer-lhe um seguimento contínuo da insuficiência cardíaca. É importante que informe o seu médico se teve ou tem algum problema grave de coração. Se desenvolve novos sintomas de insuficiência cardíaca ou pioram os atuais (por exemplo, dificuldade para respirar ou inchaço dos pés), deve poner-se em contacto imediatamente com o seu médico. O seu médico decidirá se deve deixar o tratamento com Cimzia.
Câncer
- É pouco frequente, mas foram observados casos de certos tipos de CÂNCERem pacientes tratados com Cimzia ou com outros agentes bloqueantes do TNF. As pessoas com artrite reumatoide mais grave e que padecem a doença desde há muito tempo podem ter um risco maior que a média de desenvolver um linfoma, um tipo de câncer que afeta o sistema linfático. Se está em tratamento com Cimzia, pode aumentar o risco de contrair linfoma ou outros tipos de câncer. Além disso, foram observados casos pouco frequentes de câncer de pele tipo não melanoma em pacientes que utilizam Cimzia. Informe o seu médico se durante ou após o tratamento com Cimzia aparecerem novas lesões na pele ou se as lesões existentes mudam de aparência. Há casos de câncer, incluindo tipos pouco frequentes, em crianças e adolescentes que tomavam agentes bloqueantes do TNF, os quais em algumas ocasiões terminaram em morte (ver mais abaixo “Uso em crianças e adolescentes”).
Outras doenças
- Os pacientes com doença pulmonar obstructiva crónica (DPOC) ou os que fumam muito podem ter um maior risco de padecer câncer com o tratamento de Cimzia. Se tem DPOC, ou fuma muito, deve consultar o seu médico se o tratamento com um medicamento bloqueante do TNF é apropriado no seu caso.
- Se padece uma doença do sistema nervoso, como esclerose múltipla, o seu médico decidirá se deve utilizar Cimzia.
- Em alguns pacientes, o organismo pode ser incapaz de produzir um número suficiente de células sanguíneas que ajudam o corpo a lutar contra as infecções ou de aquelas que contribuem para parar as hemorragias. Se tem febre persistente, cardenais ou sangra muito facilmente ou está muito pálido, consulte imediatamente o seu médico. O seu médico pode decidir a interrupção do tratamento com Cimzia.
- É pouco frequente, mas podem aparecer sintomas de uma doença chamada lúpus (por exemplo, erupção persistente, febre, dor nas articulações e cansaço). Contacte o seu médico se experimenta estes sintomas. O seu médico pode decidir deixar o tratamento com Cimzia.
Vacinas
- Informe o seu médico se lhe foram administradas ou lhe vão ser administradas vacinas. Algumas vacinas (vivas) não devem ser administradas enquanto estiver em tratamento com Cimzia.
- Algumas vacinas podem causar infecções. Se recebeu tratamento com Cimzia durante a gravidez, o seu bebê pode ter um maior risco de contrair tais infecções até aproximadamente cinco meses após a última dose recebida durante a gravidez. É importante que informe os médicos do seu bebê e outros profissionais de saúde sobre o tratamento com Cimzia para que eles possam decidir quando o seu bebê deve ser vacinado.
Intervenções quirúrgicas ou dentárias
- Informe o seu médico se lhe vão realizar uma intervenção quirúrgica ou dentária. Informe o cirurgião ou dentista que lhe vai realizar a intervenção de que está em tratamento com Cimzia, mostrando-lhe a Tarjeta de Información para o Paciente.
Crianças e adolescentes
O uso de Cimzia não é recomendado em pacientes menores de 18 anos de idade.
Uso de Cimzia com outros medicamentos
NÃOdeve utilizar Cimzia se está utilizando os seguintes medicamentos para tratar a artrite reumatoide:
- anakinra
- abatacept
Se tiver alguma dúvida, consulte o seu médico.
Cimzia pode ser utilizado junto com:
- metotrexato,
- corticosteroides ou
- medicamentos para a dor, incluindo os medicamentos anti-inflamatórios não esteroideos (também chamados AINE).
Informe o seu médico ou farmacêutico se está tomando, tomou recentemente ou pode ter que tomar qualquer outro medicamento.
Gravidez e lactação
Se está grávida ou em período de lactação, acredita que possa estar grávida ou tem intenção de ficar grávida, consulte o seu médico ou farmacêutico antes de utilizar este medicamento.
Cimzia só deve ser utilizado durante a gravidez se for claramente necessário. Se é uma mulher em idade fértil, consulte com o seu médico o uso de medidas anticonceptivas adequadas enquanto utilizar Cimzia. Para as mulheres que têm intenção de ficar grávidas, a contracepção pode ser considerada durante os 5 meses após a última administração de Cimzia.
Se recebeu tratamento com Cimzia durante a gravidez, o seu bebê pode ter um maior risco de contrair uma infecção. É importante que informe os médicos do seu bebê e outros profissionais de saúde sobre o tratamento com Cimzia antes de o bebê ser vacinado (para mais informações, veja a secção sobre as vacinas).
Cimzia pode ser utilizado durante a lactação.
Condução e uso de máquinas
A influência de Cimzia sobre a capacidade para conduzir e utilizar máquinas é pequena. Podem produzir-se tonturas (incluindo sensação de que a sala dá voltas, visão borrosa e cansaço) após utilizar Cimzia.
Cimzia contémacetato de sódio e cloreto de sódio
Este medicamento contém menos de 23 mg (1 mmol) de sódio por 400 mg, por lo que se considera essencialmente “isento de sódio”.
3. Como usar Cimzia
Siga exatamente as instruções de administração deste medicamento indicadas pelo seu médico ou farmacêutico. Em caso de dúvida, consulte novamente o seu médico ou farmacêutico.
Artrite reumatoide
- A dose de início para adultos com artrite reumatoide é de 400 mg administrados nas semanas 0, 2 e 4.
- Esta dose é seguida de uma dose de manutenção de 200 mg cada duas semanas. Se responder ao medicamento, o seu médico pode prescrever-lhe uma dose de manutenção alternativa de 400 mg cada 4 semanas.
- O tratamento com metotrexato é mantido durante o uso de Cimzia. Se o seu médico determinar que o metotrexato é inapropriado, Cimzia pode ser administrado apenas.
Espondiloartrite axial
- A dose de início para adultos com espondiloartrite axial é de 400 mg administrados nas semanas 0, 2 e 4.
- Esta dose é seguida de uma dose de manutenção de 200 mg cada duas semanas (desde a semana 6) ou 400 mg cada 4 semanas (desde a semana 8), como lhe indicou o seu médico. Se recebeu Cimzia durante pelo menos 1 ano e responde ao medicamento, o seu médico pode prescrever-lhe uma dose de manutenção reduzida de 200 mg cada 4 semanas.
Artrite psoriásica
- A dose de início para adultos com artrite psoriásica é de 400 mg administrados nas semanas 0, 2 e 4.
- Esta dose é seguida de uma dose de manutenção de 200 mg cada duas semanas. Se responder ao medicamento, o seu médico pode prescrever-lhe uma dose de manutenção alternativa de 400 mg cada 4 semanas.
- O tratamento com metotrexato é mantido durante o uso de Cimzia. Se o seu médico determinar que o metotrexato é inapropriado, Cimzia pode ser administrado apenas.
Psoríase em placas
- A dose inicial para adultos com psoríase em placas é de 400 mg cada 2 semanas administrada nas semanas 0, 2 e 4.
- Deve continuar com uma dose de manutenção de 200 mg cada 2 semanas ou 400 mg cada 2 semanas, segundo lhe indique o seu médico.
Como usar Cimzia
Cimzia normalmente lhe será administrado por um médico especialista ou um profissional de saúde. Cimzia lhe será administrado em uma injeção (dose de 200 mg) ou 2 injeções (dose de 400 mg) sob a pele (por via subcutânea, abreviado como: SC). Normalmente se injeta no músculo da coxa ou no abdômen.
No entanto, não deve ser injetado em zonas onde a pele esteja avermelhada ou apresente hematomas ou durezas.
Instruções para a autoinjeção de Cimzia
Após um treinamento apropriado, o seu médico também lhe pode permitir autoinjetar-se Cimzia. Leia as instruções sobre como injetar-se Cimzia no final deste prospecto.
Se o seu médico considerou que você pode injetar-se este medicamento, deve manter um seguimento com ele antes de continuar autoinjetando-se:
- transcorridas 12 semanas se tem artrite reumatoide, espondiloartrite axial ou artrite psoriásica; ou
- transcorridas 16 semanas se tem psoríase em placas.
Isso é para que o médico possa determinar se Cimzia lhe está funcionando ou deve considerar-se outro tratamento.
Se usa mais Cimzia do que deve
Se o seu médico considerou que você pode injetar-se este medicamento e acidentalmente se injeta Cimzia com mais frequência do que a prescrita, deve informar disso ao seu médico. Leve sempre consigo a Tarjeta de Información para o Paciente e a caixa do medicamento, mesmo que esteja vazia.
Se esqueceu de usar Cimzia
Se o seu médico considerou que você pode injetar-se este medicamento e esqueceu de administrar-se uma injeção, deve injetar-se a próxima dose de Cimzia assim que se lembrar. Depois, injete-se as próximas doses como lhe foi indicado. Fale depois com o seu médico e injete-se as próximas doses seguindo as instruções que lhe der.
Se interrompeu o tratamento com Cimzia
Não interrompa o tratamento com Cimzia sem consultar primeiro o seu médico.
Se tiver alguma outra dúvida sobre o uso deste medicamento, pergunte ao seu médico, farmacêutico ou enfermeiro.
4. Efeitos adversos possíveis
Tal como todos os medicamentos, este medicamento pode produzir efeitos adversos, embora nem todas as pessoas os sofram.
Informar IMEDIATAMENTEao seu médico se notar algum dos seguintes efeitos adversos:
- erupção grave, sarpullido ou outros sinais de reação alérgica (urticária)
- cara, mãos, pés inchados (angioedema)
- problemas para respirar, engolir (múltiplas causas para estes sintomas)
- dificuldade para respirar (dispnéia) com o esforço ou ao deitar-se, ou inchaço dos pés (insuficiência cardíaca)
- sintomas de distúrbios sanguíneos como febre persistente, equimoses, sangramento, palidez (pancitopenia, anemia, baixo recuento de plaquetas, baixo recuento de glóbulos brancos)
- erupções na pele graves. Estas podem aparecer como máculas ou manchas circulares de cor vermelha em forma de escarapela, frequentemente com bolhas centrais no tronco, descamação da pele, úlceras na boca, garganta, nariz, genitais e olhos e pode ser precedido por febre e sintomas semelhantes à gripe (síndrome de Stevens-Johnson).
Informar ao seu médico QUANTO ANTESse notar algum dos seguintes efeitos adversos:
- sinais de infecção como febre, mal-estar, feridas, problemas dentários, ardor ao urinar
- sensação de fraqueza ou cansaço
- tosse
- formigamento
- entorpecimento
- visão dupla
- fraqueza nos braços ou pernas
- golpes ou feridas que não se curam
Os sintomas descritos anteriormente podem dever-se a alguns dos efeitos adversos listados a seguir, os quais foram observados com Cimzia:
Frequentes (podem afetar até 1 de cada 10 pacientes):
- infecções bacterianas em qualquer localização (acumulação de pus)
- infecções virais (incluindo herpes labial, herpes zóster e gripe)
- febre
- pressão arterial alta
- erupção ou prurido
- dores de cabeça (incluindo enxaqueca)
- anomalias sensoriais como entorpecimento, formigamento, sensação de queimadura
- sensação de fraqueza e mal-estar geral
- dor
- distúrbios sanguíneos
- problemas hepáticos
- reações no local da injeção
- náusea
Pouco frequentes (podem afetar até 1 de cada 100 pacientes):
- doenças alérgicas incluindo rinite e reações alérgicas ao medicamento (incluindo choque anafilático)
- anticorpos dirigidos contra tecido normal
- câncer do sistema linfático e sangue como linfoma e leucemia
- tumores sólidos
- cânceres de pele, lesões de pele precancerosas
- tumores benignos (não cancerosos) e quistes (incluindo os de pele)
- problemas cardíacos incluindo músculo cardíaco debilitado, insuficiência cardíaca, infarto do miocárdio, mal-estar ou pressão no peito, ritmo cardíaco anormal incluindo batimentos cardíacos irregulares
- edema (inchaço na face ou pernas)
- sintomas de lúpus (doença imunológica que afeta o tecido conjuntivo): dor articular, erupções cutâneas, fotosensibilidade e febre
- inflamação dos vasos sanguíneos
- sepsis (infecção grave que pode dar lugar a uma falha orgânica, choque ou morte)
- infecção tuberculosa
- infecções por fungos (ocorrem quando a capacidade para lutar contra as infecções está reduzida)
- distúrbios respiratórios e inflamação (incluindo asma, respiração entrecortada, tosse, bloqueio de seios, pleurite ou dificuldade para respirar)
- problemas de estômago incluindo acumulação de fluido abdominal, úlceras (incluindo úlceras bucais), perfuração, distensão, inflamação, ardor, desconforto no estômago, secura da boca
- problemas biliares
- problemas musculares incluindo aumento de enzimas do músculo
- mudanças nos níveis de diferentes sais no sangue
- mudanças nos níveis de colesterol e lípidos no sangue
- coágulos sanguíneos nas veias ou pulmões
- hemorragia ou equimoses
- mudanças no número de células sanguíneas, incluindo baixo número de glóbulos vermelhos (anemia), baixo número de plaquetas, aumento do número de plaquetas
- nódulos linfáticos inchados
- sintomas de tipo gripal, calafrios, percepção da temperatura alterada, suores noturnos, rubor
- ansiedade e distúrbios do humor como depressão, distúrbios do apetite, mudanças de peso
- vertigem (tontura)
- zumbido nos ouvidos
- desmaio, incluindo perda da consciência
- distúrbios nos nervos das extremidades incluindo sintomas de entorpecimento, formigamento, sensação de queimadura, tontura, tremor
- distúrbios da pele como um novo episódio ou piora da psoríase, inflamação da pele (como eczema), distúrbios da glândula sudorípara, úlceras, fotosensibilidade, acne, perda de cabelo, mudanças de coloração da pele, separação das unhas, pele seca e feridas
- problemas de cicatrização
- problemas urinários e renais incluindo função renal alterada, sangue na urina e alterações urinárias
- distúrbios do ciclo menstrual (período mensal) incluindo falta de sangramento ou muito sangramento, ou sangramento irregular
- distúrbios da mama
- inflamação do olho e da pálpebra, alterações da visão, problemas lacrimais
- aumento de alguns parâmetros sanguíneos (aumento da fosfatase alcalina sanguínea)
- tempos de coagulação prolongados
Raros (podem afetar até 1 de cada 1.000 pacientes):
- câncer gastrointestinal, melanoma
- inflamação do pulmão (doença pulmonar intersticial, inflamação pulmonar)
- infarto cerebral, bloqueio dos vasos sanguíneos (arteriosclerose), má circulação sanguínea que provoca entorpecimento e palidez dos dedos dos pés e das mãos (fenômeno de Raynaud), manchas roxas, decoloração da pele, pequenas veias perto da superfície da pele podem tornar-se visíveis
- inflamação do pericárdio
- arritmia cardíaca
- agrandamento do baço
- aumento da massa dos glóbulos vermelhos
- morfolgia anormal dos glóbulos brancos
- formação de pedras na vesícula biliar
- problemas renais (incluindo nefrite)
- distúrbios imunológicos como sarcoidose (erupção, dor articular, febre), doença do soro, inflamação do tecido gordo, edema angioneurótico (inchaço dos lábios, face, garganta)
- distúrbios da tireoide (bócio, cansaço, perda de peso)
- aumento dos níveis de ferro no corpo
- aumento nos níveis sanguíneos de ácido úrico
- tentativa de suicídio, distúrbio mental, delírio
- inflamação do nervo auditivo, da visão, ou da face, alteração da coordenação ou do equilíbrio
- aumento da motilidade gastrointestinal
- fístula (conduto que comunica um órgão com outro) (em qualquer zona)
- alterações da boca incluindo dor ao engolir
- descamação da pele, distúrbios vesiculares, distúrbios na textura do cabelo
- disfunção sexual
- crise
- piora de uma doença chamada dermatomiosite (que aparece como fraqueza muscular acompanhada de uma erupção cutânea)
- síndrome de Stevens-Johnson (uma doença grave da pele na qual os primeiros sintomas incluem mal-estar geral, febre, dor de cabeça e erupção)
- erupção inflamatória na pele (eritema multiforme)
- reações liquenoides (erupção cutânea pruriginosa roxa-morada e/ou linhas grossas branco-acinzentadas nas mucosas)
Não conhecida (a frequência não pode ser estimada a partir dos dados disponíveis):
- esclerose múltipla*
- síndrome de Guillain-Barré*
- carcinoma de células de Merkel (um tipo de câncer de pele)*
- sarcoma de Kaposi (um câncer pouco comum relacionado com a infecção pelo vírus do herpes humano tipo 8. O sarcoma de Kaposi geralmente se manifesta com maior frequência como lesões cutâneas de cor púrpura).
*Estes eventos foram relacionados com esta classe de medicamentos, mas a incidência em Cimzia é desconhecida.
Outros efeitos adversos
Quando Cimzia foi utilizado para tratar outras doenças, ocorreram os seguintes efeitos adversos pouco frequentes:
- estenose gastrointestinal (estreitamento de parte do aparelho digestivo).
- obstrução gastrointestinal (bloqueio do aparelho digestivo).
- declínio da saúde física geral.
- aborto espontâneo.
- azoospermia (falta de produção de esperma).
Comunicação de efeitos adversos
Se experimentar qualquer tipo de efeito adverso, consulte o seu médico, farmacêutico ou enfermeiro, mesmo que se trate de efeitos adversos que não aparecem neste prospecto. Também pode comunicá-los diretamente através do sistema nacional de notificação incluído no Apêndice V.
Ao comunicar efeitos adversos, você pode contribuir para fornecer mais informações sobre a segurança deste medicamento.
5. Conservação de Cimzia
Mantenha este medicamento fora da vista e do alcance das crianças.
Não utilize este medicamento após a data de validade que aparece no envase após CAD e na seringa após EXP. A data de validade é o último dia do mês que se indica.
Conservar na geladeira (2 °C-8 °C).
Não congelar.
Conservar a seringa pré-carregada no embalagem exterior para protegê-la da luz.
As seringas pré-carregadas podem ser conservadas a temperatura ambiente (não superior a 25 °C) durante um período único máximo de 10 dias protegidas da luz. Ao final deste período as seringas pré-carregadas devem ser usadas ou descartadas.
Não use este medicamento se a solução estiver descolorida, turva ou se puder ver partículas nela.
Os medicamentos não devem ser jogados nos esgotos nem na lixeira. Pergunte ao seu farmacêutico como se livrar dos envases e dos medicamentos que já não precisa. Dessa forma, você ajudará a proteger o meio ambiente.
6. Conteúdo do envase e informação adicional
Composição de Cimzia
- O princípio ativo é certolizumab pegol. Cada seringa precarregada contém 200 mg de certolizumab pegol em 1 ml.
- Os outros componentes são: acetato de sódio, cloreto de sódio e água para preparações injetáveis (ver “Cimzia contém acetato de sódio e cloreto de sódio” na seção 2).
Aspecto do produto e conteúdo do envase
Cimzia é fornecido como uma solução injetável pronta para uso em uma seringa precarregada. A solução é clara a opalescente, incolora a amarelada.
Um envase de Cimzia contém:
- duas seringas precarregadas que contêm uma solução e
- duas toalhetas com álcool (para limpar as áreas escolhidas para a injeção).
Estão disponíveis envases com 2 seringas precarregadas e 2 toalhetas com álcool, envases múltiplos que contêm 6 seringas precarregadas (3 envases de 2) e 6 toalhetas com álcool (3 envases de 2) e envases múltiplos que contêm 10 seringas precarregadas (5 envases de 2) e 10 toalhetas com álcool (5 envases de 2).
Pode ser que apenas alguns tamanhos de envases sejam comercializados.
Título da autorização de comercialização
UCB Pharma SA
Allée de la Recherche 60
B-1070 Bruxelas
Bélgica
Responsável pela fabricação
UCB Pharma S.A.
Chemin du Foriest
B-1420 Braine l'Alleud
Bélgica
Pode solicitar mais informações sobre este medicamento dirigindo-se ao representante local do titular da autorização de comercialização:
Bélgica UCB Pharma S.A./NV Tel/Tél: + 32 / (0)2 559 92 00 | Lituânia UCB Pharma Oy Finlândia Tel: + 358 9 2514 4221 (Finlândia) |
| Luxemburgo UCB Pharma S.A.//NV Tél/Tel: + 32 / (0)2 559 92 00 (Bélgica) |
República Tcheca UCB s.r.o. Tel: + 420 221 773 411 | Hungria UCB Hungria Kft. Tel.: + 36-(1) 391 0060 |
Dinamarca UCB Nordic A/S Tlf.: + 45 / 32 46 24 00 | Malta Pharmasud Ltd. Tel: + 356 / 21 37 64 36 |
Alemanha UCB Pharma GmbH Tel: + 49 / (0)2173 48 4848 | Países Baixos UCB Pharma B.V. Tel.: + 31 / (0)76-573 11 40 |
Estônia UCB Pharma Oy Finlândia Tel: + 358 9 2514 4221 (Finlândia) | Noruega UCB Nordic A/S Tlf: + 47 / 67 16 5880 |
Grécia UCB Α.Ε. Τηλ: + 30 / 2109974000 | Áustria UCB Pharma GmbH Tel: + 43-(0)1 291 80 00 |
Espanha UCB Pharma S.A. Tel: + 34 / 91 570 34 44 | Polônia/ VEDIM Sp. z o.o. UCB Pharma Sp. z o.o. Tel.: + 48 22 696 99 20 |
França UCB Pharma S.A. Tél: + 33 / (0)1 47 29 44 35 | Portugal UCB Pharma (Produtos Farmacêuticos), Lda Tel: + 351 / 21 302 5300 |
Croácia Medis Adria d.o.o. Tel: +385 (0) 1 230 34 46 | Romênia UCB Pharma Romênia S.R.L. Tel: + 40 21 300 29 04 |
Irlanda UCB (Pharma) Irlanda Ltd. Tel: + 353 / (0)1-46 37 395 | Eslovênia Medis, d.o.o. Tel: + 386 1 589 69 00 |
Islândia Vistor hf. Tel: + 354 535 7000 | República Eslovaca UCB s.r.o., organizačná zložka Tel: + 421 (0) 2 5920 2020 |
Itália UCB Pharma S.p.A. Tel: + 39 / 02 300 791 | Finlândia UCB Pharma Oy Finlândia Puh/Tel: + 358 9 2514 4221 |
Chipre Lifepharma (Z.A.M.) Ltd Τηλ: + 357 22 056300 | Suécia UCB Nordic A/S Tel: + 46 / (0) 40 29 49 00 |
Letônia UCB Pharma Oy Finlândia Tel: + 358 9 2514 4221 |
Data da última revisão deste prospecto:{MM/AAAA}.
Outras fontes de informação
A informação detalhada sobre este medicamento está disponível no site da Agência Europeia de Medicamentos: http://www.ema.europa.eu/.
<-------------------------------------------------------------------------------------------------------------------------
INSTRUÇÕES PARA A INJEÇÃO DE CIMZIA COM UMA SERINGA PRECARREGADA
Após um treinamento apropriado, você pode se autoinjetar ou outra pessoa, por exemplo, um familiar ou amigo, pode injetar. As seguintes instruções explicam como injetar Cimzia. Leia-as com atenção e siga-as passo a passo. Seu médico ou outro profissional de saúde o ensinará como se autoinjetar. Não tente se autoinjetar antes de ter certeza de que entendeu como preparar e administrar a injeção.
Não misture o conteúdo na mesma seringa ou frasco com qualquer outro medicamento.
- Preparação
- Retire o envase de Cimzia da geladeira.
- Se o(s) selo(s) não estiver(em) ou estiver(em) quebrado(s) – não o use e entre em contato com seu farmacêutico.
- Retire os seguintes elementos da caixa de Cimzia e coloque-os sobre uma superfície plana e limpa:
- - Uma ou duas seringa(s) precarregada(s), dependendo da dose prescrita.
- - Uma ou duas toalhetas com álcool
- Verifique a data de validade da seringa e da caixa. Não use Cimzia após a data de validade que aparece na caixa após CAD e na seringa após EXP. A data de validade é o último dia do mês indicado.
- Deixe a seringa precarregada atingir a temperatura ambiente. Isso levará 30 minutos. Isso ajudará a reduzir o desconforto no momento da injeção.
- Não aqueça a seringa precarregada - deixe que atinja a temperatura ambiente sozinha.
- Não retire o capuchão até que esteja pronto para se autoinjetar.
- Lave as mãos meticulosamente.
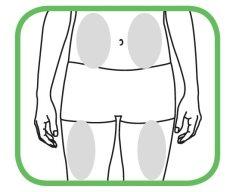
- Escolha e preparação da zona de injeção
- Escolha uma zona em sua coxa ou abdômen.
|
- Cada nova injeção deve ser aplicada em uma zona separada da zona da última injeção.
- Nunca se injete em uma zona onde a pele esteja vermelha, contundida ou endurecida.
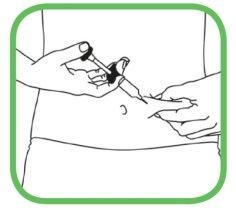
- Limpe a zona de injeção com a toalheta com álcool que acompanha, seguindo um movimento circular de dentro para fora.
- Não toque novamente nessa zona antes da injeção.
- Injeção
- Não agite a seringa.
Verifique o medicamento no corpo da seringa.
- Não use se a solução estiver descolorida, turva ou se puder ver partículas nela.
- Pode observar bolhas de ar. Isso é normal. Injetar subcutaneamente uma solução que contém bolhas de ar é inofensivo.
- Retire o capuchão da agulha sem incliná-lo, com cuidado para não tocar a agulha ou deixá-la em contato com qualquer superfície. Não dobre a agulha.
- Injete nos 5 minutos seguintes após remover o capuchão da agulha.
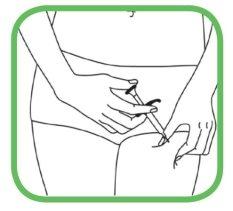
- Pegue suavemente a zona de pele limpa com uma mão e segure-a com firmeza.
|
- Com a outra mão, segure a seringa formando um ângulo de 45 graus com relação à pele.
- Com um movimento curto e rápido, insira toda a agulha na pele.
- Empurre o êmbolo para injetar a solução. Pode levar cerca de 10 segundos para esvaziar a seringa.
- Quando a seringa estiver vazia, retire a agulha da pele com cuidado, com o mesmo ângulo que quando foi inserida.
- Solte a zona de pele que estava segurando com a primeira mão.
- Usando um pouco de algodão, pressione sobre o local da injeção por alguns segundos.
- Não esfregue o local da injeção.
- Se necessário, pode cobrir a zona da injeção com uma bandagem adesiva.
- Após o uso
- Não reutilize a seringa nem recoloque o capuchão na agulha.
- Após a injeção, descarte imediatamente a(s) seringa(s) usada(s) em um contenedor especial, como lhe explicou seu médico, enfermeiro ou farmacêutico.
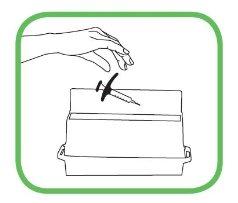
- Mantenha o contenedor fora da vista e do alcance das crianças.
- Se precisar realizar uma segunda injeção, como prescrito por seu médico, repita o processo de injeção começando no passo 2.
- País de registo
- Substância ativa
- Requer receita médicaSim
- Fabricante
- Esta informação é apenas para referência e não constitui aconselhamento médico. Consulte sempre um médico antes de tomar qualquer medicamento. A Oladoctor não se responsabiliza por decisões médicas baseadas neste conteúdo.
- Alternativas a CIMZIA 200 mg SOLUÇÃO INJETÁVEL EM SERINGA PREENCHIDAForma farmacêutica: INJETÁVEL, 200 mg/mlSubstância ativa: certolizumab pegolFabricante: Ucb PharmaRequer receita médicaForma farmacêutica: INJETÁVEL, 200 mg/mlSubstância ativa: certolizumab pegolFabricante: Ucb PharmaRequer receita médicaForma farmacêutica: INJETÁVEL, 20 mgSubstância ativa: adalimumabFabricante: Amgen Europe B.V.Requer receita médica
Médicos online para CIMZIA 200 mg SOLUÇÃO INJETÁVEL EM SERINGA PREENCHIDA
Avaliação de posologia, efeitos secundários, interações, contraindicações e renovação da receita de CIMZIA 200 mg SOLUÇÃO INJETÁVEL EM SERINGA PREENCHIDA – sujeita a avaliação médica e regras locais.