
ADYNOVI 3000 UI/5 mL PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL
Pergunte a um médico sobre a prescrição de ADYNOVI 3000 UI/5 mL PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL

Como usar ADYNOVI 3000 UI/5 mL PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL
Introdução
Prospecto: informação para o utilizador
ADYNOVI 250 UI/5 ml pó e dissolvente para solução injetável
ADYNOVI 500 UI/5 ml pó e dissolvente para solução injetável
ADYNOVI 1 000 UI/5 ml pó e dissolvente para solução injetável
ADYNOVI 2 000 UI/5 ml pó e dissolvente para solução injetável
ADYNOVI 3000 UI/5 ml pó e dissolvente para solução injetável
rurioctocog alfa pegol (fator VIII humano de coagulação recombinante pegilado)
Este medicamento está sujeito a acompanhamento adicional, o que agilizará a detecção de nova informação sobre a sua segurança. Pode contribuir comunicando os efeitos adversos que possa ter. A parte final da seção 4 inclui informação sobre como comunicar efeitos adversos.
Leia todo o prospecto atentamente antes de começar a usar este medicamento, porque contém informação importante para si.
- Conserva este prospecto, porque pode ter que voltar a lê-lo.
- Se tiver alguma dúvida, consulte o seu médico.
- Este medicamento foi prescrito apenas para si, e não deve dá-lo a outras pessoas, mesmo que tenham os mesmos sintomas que si, porque pode prejudicá-las.
- Se experimentar efeitos adversos, consulte o seu médico, mesmo que se trate de possíveis efeitos adversos que não aparecem neste prospecto. Ver seção 4.
Conteúdo do prospecto
- O que é ADYNOVI e para que é utilizado
- O que precisa saber antes de começar a usar ADYNOVI
- Como usar ADYNOVI
- Possíveis efeitos adversos
- Conservação de ADYNOVI
- Conteúdo do envase e informação adicional
1. O que é ADYNOVI e para que é utilizado
ADYNOVI contém o princípio ativo rurioctocog alfa pegol, fator VIII humano de coagulação pegilado. O fator VIII humano de coagulação foi modificado para prolongar a duração da sua ação. O fator VIII é necessário para que o sangue forme coágulos e para deter as hemorragias. Nos pacientes com hemofilia A (falta congénita de fator VIII) não está presente ou não actua de forma correcta.
ADYNOVI é utilizado para o tratamento e a prevenção da hemorragia em pacientes a partir de 12 anos com hemofilia A (um distúrbio hemorrágico hereditário provocado pela ausência de fator VIII)
2. O que precisa saber antes de começar a usar ADYNOVI
Não use ADYNOVI
- se é alérgico a rurioctocog alfa pegol, octocog alfa ou a algum dos outros componentes deste medicamento (incluídos na seção 6)
- se é alérgico a proteínas de rato ou hamster
Se tiver alguma dúvida, consulte o seu médico.
Advertências e precauções
É importante manter um registo do número do lote do seu ADYNOVI. Por isso, cada vez que obtiver um novo envase de ADYNOVI, anote a data e o número do lote (que está no cartonagem após a abreviatura “Lote”) e guarde esta informação num local seguro.
Consulte o seu médico antes de começar a usar ADYNOVI.
Existe um risco muito pequeno de que sofra uma reação anafiláctica (uma reação alérgica grave repentina) a ADYNOVI. Deve conhecer os sinais iniciais das reações alérgicas, tais como erupção, habão urticarial, ronchas, picor generalizado, inchação dos lábios e língua, dificuldade para respirar, ruídos ao respirar (sibilância), opressão no peito, sensação de malestar geral e tontura. Estes poderiam ser sintomas iniciais de choque anafiláctico. Outros sintomas podem incluir tontura intensa, perda de consciência e muita dificuldade para respirar.
Se aparecer algum destes sintomas, interrompa de forma imediata a injeção e consulte o seu médico. Os sintomas graves, como a dificuldade para respirar e o (quase)desmaio, necessitam de tratamento urgente.
Se sofrer de alguma doença cardíaca, informe o seu médico, porque existe um risco maior de complicações por formação de coágulos no sangue.
Pacientes que desenvolvem inibidores do fator VIII
A formação de inibidores (anticorpos) é uma complicação conhecida que pode ocorrer durante o tratamento com todos os medicamentos compostos por fator VIII. Estes inibidores, em especial em grandes quantidades, impedem que o tratamento funcione de forma correcta, por isso si e o seu filho serão cuidadosamente controlados por si desenvolverem ditos inibidores. Se a sua hemorragia ou a do seu filho não estiver a ser controlada com ADYNOVI, consulte o seu médico de forma imediata.
Complicações relacionadas com o catéterSe necessitar utilizar um catéter venoso central (CVC), deve ter em conta o risco de complicações relacionadas com dito dispositivo, tais como infecções locais, presença de bactérias no sangue e trombose na zona do catéter.
Crianças e adolescentes
ADYNOVI só pode ser utilizado em adolescentes e adultos (maiores de 12 anos). As advertências e precauções que se indicam também se aplicam aos adolescentes
Outros medicamentos e ADYNOVI
Informe o seu médico se está a utilizar, utilizou recentemente ou possa ter que utilizar qualquer outro medicamento.
Gravidez e amamentação
Se está grávida ou em período de amamentação, acredita que possa estar grávida ou tem intenção de engravidar, consulte o seu médico antes de utilizar este medicamento. A hemofilia A afeta apenas raramente as mulheres. Por isso, não se dispõe de experiência em relação ao uso de ADYNOVI durante a gravidez e a amamentação
Condução e uso de máquinas
A influência de ADYNOVI sobre a capacidade para conduzir e utilizar máquinas é nula ou insignificante.
ADYNOVI contém sódio
ADYNOVI contém até 12,42 mg de sódio (componente principal do sal de mesa/para cozinhar) por frasco. Isto equivale a 0,62 % da ingestão diária máxima de sódio recomendada para um adulto. Dependendo do seu peso corporal e da sua dose de ADYNOVI, pode receber mais de um frasco. Isto deve ser tido em conta se si segue uma dieta baixa em sal.
3. Como usar ADYNOVI
O tratamento com ADYNOVI será iniciado e supervisionado por um médico com experiência no tratamento de pacientes com hemofilia A.
O seu médico calculará a sua dose de ADYNOVI em função do seu estado e peso corporal e de se é utilizado para a prevenção ou para o tratamento da hemorragia. A frequência da administração dependerá de como actua ADYNOVI no seu caso. O tratamento de substituição com ADYNOVI é normalmente um tratamento de por vida.
Siga exactamente as instruções de administração deste medicamento indicadas pelo seu médico. Em caso de dúvida, consulte de novo o seu médico.
Prevenção da hemorragia
A dose habitual de ADYNOVI é de 40 a 50 UI por kg de peso corporal, administrada 2 vezes por semana.
Tratamento da hemorragia
A dose de ADYNOVI é calculada em função do seu peso corporal e dos níveis de fator VIII que se querem alcançar. Os níveis de fator VIII a alcançar dependerão da gravidade e da localização da hemorragia.
Consulte o seu médico se acredita que o efeito de ADYNOVI é insuficiente.
O seu médico realizará os análises de laboratório adequados para se certificar de que tem os níveis adequados de fator VIII. Isto é, especialmente, importante se se vai submeter a cirurgia maior.
Uso em crianças e adolescentes
ADYNOVI só pode ser utilizado em adolescentes e adultos (maiores de 12 anos). A dose para os adolescentes é calculada também segundo o peso corporal e é a mesma que para os adultos.
Como se administra ADYNOVI
Normalmente o médico ou o enfermeiro injetam ADYNOVI numa veia (via intravenosa). Si ou qualquer outra pessoa podem administrar também a injeção de ADYNOVI, mas apenas após receber a formação adequada. As instruções detalhadas para a autoadministração são descritas no final deste prospecto.
Se usar mais ADYNOVI do que deve
Siga exactamente as instruções de administração de ADYNOVI indicadas pelo seu médico. Consulte o seu médico se tiver dúvidas. Se se injetar uma dose maior de ADYNOVI do que a recomendada, consulte com o seu médico o mais breve possível.
Se esquecer de usar ADYNOVI
Não se injete uma dose dupla para compensar as doses esquecidas. Administre a próxima injeção como está estabelecido e continue como o seu médico indicou.
Se interromper o tratamento com ADYNOVI
Não deixe de usar ADYNOVI sem consultar o seu médico.
Se tiver alguma outra dúvida sobre o uso deste medicamento, pergunte ao seu médico.
4. Possíveis efeitos adversos
Como todos os medicamentos, este medicamento pode produzir efeitos adversos, embora nem todas as pessoas os sofram.
Se se produzirem reações alérgicas (anafilácticas) graves e repentinas, deve parar de forma imediata a injeção. Contacte com o seu médico de forma imediatase tiver algum dos seguintes sintomas iniciais das reações alérgicas:
- erupção, habão urticarial, ronchas, picor generalizado,
- inchação dos lábios e língua,
- dificuldade para respirar, ruídos ao respirar, opressão no peito,
- sensação de malestar geral,
- tontura e perda de consciência.
Os sintomas graves, como a dificuldade para respirar e o (quase)desmaio, necessitam de tratamento urgente imediato
Nos pacientes que receberam tratamento prévio com fator VIII (mais de 150 dias de tratamento), podem formar-se anticorpos inibidores (ver seção 2) com pouca frequência (menos de 1 de cada 100 pacientes). Se isto acontecer, o medicamento que toma pode deixar de funcionar correctamente e si pode sofrer uma hemorragia persistente. Nesse caso, contacte com o seu médico de forma imediata.
Efeitos adversos muito frequentes(podem afectar a mais de 1 de cada 10 pessoas)
Cefaleia
Efeitos secundários frequentes(podem afectar até 1 de cada 10 pessoas)
Náuseas
Diarreia
Erupção
Tontura
Habão urticarial
Efeitos adversos pouco frequentes(podem afectar até 1 de cada 100 pessoas)
Rubefacção, reação alérgica (hipersensibilidade)
Inibidores do fator VIII (para pacientes que tenham recebido tratamento anterior com fator VIII (mais de 150 dias de tratamento))
Aumento de algum tipo de glóbulos brancos
Reação à perfusão
Vermelhidão do olho
Reação adversa ao fármaco da pele
Outros efeitos adversos em crianças
Espera-se que a frequência, o tipo e a gravidade das reações adversas em crianças sejam os mesmos que nos adultos.
Comunicação de efeitos adversos
Se experimentar qualquer tipo de efeito adverso, consulte o seu médico, mesmo que se trate de possíveis efeitos adversos que não aparecem neste prospecto. Também pode comunicá-los directamente através do sistema nacional de notificação incluído no Apêndice V. Mediante a comunicação de efeitos adversos, si pode contribuir para proporcionar mais informação sobre a segurança deste medicamento.
5. Conservação de ADYNOVI
Mantenha este medicamento fora da vista e do alcance das crianças.
Não utilize este medicamento após a data de validade que aparece na etiqueta e na caixa após CAD. A data de validade é o último dia do mês que se indica.
Conservar em frigorífico (entre 2 ºC e 8 ºC).
Não congelar.
Conservar blister no embalagem exterior para protegê-lo da luz.
Durante o seu período de validade, o frasco de pó pode ser conservado a temperatura ambiente (até 30 ºC) durante um período único que não ultrapasse os 3 meses. Nesse caso, este medicamento caduca ao final deste período de 3 meses ou na data de validade impressa no frasco de produto, o que ocorrer antes. Por favor, anote no embalagem exterior a data de finalização do período de conservação a temperatura ambiente de 3 meses. O medicamento não se pode refrigerar de novo após a conservação a temperatura ambiente. Não refrigerar o medicamento após a preparação.
Utilizar o medicamento num prazo de 3 horas após a dissolução completa do pó.
Este medicamento é para um só uso. Eliminar a solução não utilizada de forma adequada
Os medicamentos não se devem deitar por os esgotos nem para o lixo. Pergunte ao seu farmacêutico como se desfazer dos embalagens e dos medicamentos que já não precisa. Desta forma, ajudará a proteger o meio ambiente.
6. Conteúdo do envase e informação adicional
Composição de ADYNOVI
- O princípio ativo é rurioctocog alfa pegol (fator VIII humano de coagulação produzido mediante tecnologia de DNA recombinante). Cada frasco de pó contém nominalmente 250, 500, 1 000, 2 000 ou 3000 UI de rurioctocog alfa pegol.
- O frasco de dissolvente contém 5 ml de água para preparações injetáveis.
- Os demais ingredientes são manitol, dihidrato de trealose, histidina, glutatión, cloreto de sódio, cloreto de cálcio dihidratado, tris(hidroximetil)aminometano e polissorbato 80. Ver seção 2 “ADYNOVI contém sódio”.
Aspecto do produto e conteúdo do envase
ADYNOVI é fornecido sob a forma de pó e dissolvente para solução injetável (pó para solução injetável). O pó é um pó de cor branca a esbranquiçada que se desmenuza. O dissolvente é uma solução incolor e transparente. Depois da sua reconstituição, a solução é transparente, incolor e livre de partículas estranhas.
Título da autorização de comercialização
Baxalta Innovations GmbH
Industriestrasse 67
A-1221 Viena
Fabricante
Baxalta Belgium Manufacturing SA
Boulevard René Branquart 80
B-7860 Lessines
Bélgica
Podem solicitar mais informações sobre este medicamento dirigindo-se ao representante local do título da autorização de comercialização:
Bélgica/Bélgica/Bélgica Takeda Belgium NV Tel/Tél: +32 2 464 06 11 | Lituânia Takeda, UAB Tel: +370 521 09 070 |
| Luxemburgo/Luxemburgo Takeda Belgium NV Tel/Tél: +32 2 464 06 11 |
República Checa Takeda Pharmaceuticals Czech Republic s.r.o. Tel: +420 234 722 722 | Hungria Takeda Pharma Kft. Tel: +36 1 270 7030 |
Dinamarca Takeda Pharma A/S Tlf: +45 46 77 10 10 | Malta Τakeda HELLAS S.A. Tel: +30 210 6387800 |
Alemanha Takeda GmbH Tel: +49 (0)800 825 3325 | Países Baixos Takeda Nederland B.V. Tel: +31 20 203 5492 |
Estônia Takeda Pharma AS Tel: +372 6177 669 | Noruega Takeda AS Tlf: +47 800 800 30 |
Grécia Τakeda ΕΛΛΑΣ Α.Ε. Tηλ: +30 210 6387800 | Áustria Takeda Pharma Ges.m.b.H. Tel: +43 (0) 800-20 80 50 [email protected] |
Espanha Takeda Farmacêutica Espanha, S.A Tel: +34 917 90 42 22 | Polônia Takeda Pharma Sp. z o.o. tel: +48223062447 |
França Takeda France SAS Tel. + 33 1 40 67 33 00 | Portugal Takeda Farmacêuticos Portugal, Lda. Tel: + 351 21 120 1457 |
Croácia Takeda Pharmaceuticals Croatia d.o.o. Tel: +385 1 377 88 96 | Romênia Takeda Pharmaceuticals SRL Tel: +40 21 335 03 91 |
Irlanda Takeda Products Ireland Ltd Tel: 1800 937 970 |
|
Islândia Vistor hf. Sími: +354 535 7000 | República Eslovaca Takeda Pharmaceuticals Slovakia s.r.o. Tel: +421 (2) 20 602 600 |
Itália Takeda Italia S.p.A. Tel: +39 06 502601 | Finlândia Takeda Oy Puh/Tel: 0800 774 051 |
Chipre Proton Medical (Chipre) Ltd Tηλ: +357 22866000 | Suécia Takeda Pharma AB Tel: 020 795 079 |
Letônia Takeda Latvia SIA Tel: +371 67840082 | Reino Unido (Irlanda do Norte) Takeda UK Ltd Tel: +44 (0) 2830 640 902 |
Data da última revisão deste prospecto:
A informação detalhada deste medicamento está disponível no site da Agência Europeia de Medicamentos: http://www.ema.europa.eu/
Instruções para a preparação e administração
ADYNOVI não deve ser misturado com outros medicamentos ou dissolventes.
Recomenda-se fortemente registrar o nome e o número do lote do produto cada vez que se administra ADYNOVI. O blister tem etiquetas que podem ser removidas.
Instruções para a reconstituição
- Não utilizar após a data de validade que aparece nas etiquetas e no envase.
- Não utilizar se a tampa do blister não estiver perfeitamente selada.
- Não refrigerar o medicamento após a preparação.
- Se o medicamento ainda estiver na geladeira, pegue o blister selado (contém os frascos de pó e dissolvente pré-montados no sistema para reconstituição) e espere até que atinja a temperatura ambiente (entre 15 °C e 25 °C).
- Lave as mãos com sabão e água morna.
- Abrir o blister de ADYNOVI removendo a tampa. Retire o sistema BAXJECT III do blister.
- Coloque o frasco de pó em uma superfície plana com o frasco de dissolvente em cima (Figura 1). O frasco de dissolvente tem uma linha azul. Não remova o protetor azul até que seja indicado mais adiante.
- Enquanto segura o frasco de pó com uma mão no sistema BAXJECT III, pressione com força o frasco de dissolvente com a outra mão até que o sistema fique totalmente contraído e o dissolvente entre no frasco de pó (Figura 2). Não incline o sistema até que a transferência esteja concluída.
- Verifique se a transferência do dissolvente foi concluída. Agite suavemente até que todo o material seja dissolvido (Figura 3). Verifique se o pó está completamente dissolvido; se não estiver, toda a solução reconstituída não passará pelo filtro do dispositivo. O medicamento se dissolve rapidamente (normalmente em menos de 1 minuto). Após a reconstituição, a solução deve ser transparente, incolor e livre de partículas estranhas.
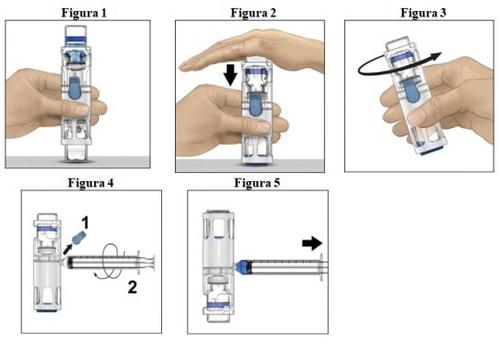
Instruções para a injeção
Durante a administração, é necessária uma técnica antiséptica (em condições de limpeza e poucos germes).
Nota importante:
- Examine a solução preparada para detectar partículas ou mudanças de cor antes da administração (a solução deve ser transparente, incolor e livre de partículas).
Não utilize se a solução não for totalmente transparente ou o produto não estiver dissolvido completamente.
- Remova o protetor azul do dispositivo BAXJECT III (Figura 4). Não introduza ar na seringa. Conecte a seringa ao BAXJECT III. Recomenda-se utilizar uma seringa Luer-lock.
- Vire o sistema (o frasco de pó estará agora na parte superior). Introduza a solução reconstituída na seringa, puxando o êmbolo para trás lentamente (Figura 5).
- Desconecte a seringa, conecte uma agulha de palomita à seringa e injete a solução reconstituída em uma veia. A solução deve ser administrada lentamente, a uma velocidade determinada de acordo com o nível de confort do paciente, que não supere os 10 ml por minuto. (Ver seção 4 "Possíveis efeitos adversos").
- Elimine a solução não utilizada de forma apropriada.
Esta informação é destinada apenas a profissionais do setor de saúde
Tratamento a demanda
Em caso de episódios de sangramento seguintes, a atividade de fator VIII não deve ser inferior ao nível de atividade plasmática dada (em % da normalidade ou UI/dl) no período correspondente. A seguinte tabela pode ser utilizada como guia posológica em cirurgia e nos episódios de sangramento:
Tabela 1: Guia posológica de dosagem em episódios de sangramento e cirurgia
Grau de sangramento/tipo de procedimento cirúrgico | Nível de fator VIII requerido (% ou UI/dl) | Frequência das doses (horas)/duração do tratamento (dias) |
HemorragiaHemartrose incipiente ou sangramento muscular ou oral. | 20 – 40 | Repetir as injeções a cada 12 a 24 horas. Pelo menos 1 dia, até que o episódio de sangramento se resolva, com base na dor, ou até que ocorra a cicatrização. |
Hemartrose mais extensa, sangramento muscular ou hematoma | 30 – 60 | Repetir as injeções a cada 12 a 24 horas durante 3–4 dias ou mais até que a dor e a discapacidade aguda se resolvam. |
Hemorragias potencialmente mortais. | 60 – 100 | Repetir as injeções a cada 8 a 24 horas até que o risco desapareça. |
Cirurgia Menor Incluindo extração dental | 30 – 60 | A cada 24 horas, pelo menos 1 dia, até que ocorra a cicatrização. |
Maior | 80 – 100 (pré e pós-operatório) | Repetir as injeções a cada 8 a 24 horas até que a ferida cicatrize de forma adequada e, em seguida, continue o tratamento por pelo menos mais 7 dias para manter uma atividade de fator VIII de 30 % a 60 % (UI/dl). |
Profilaxia
Para a profilaxia a longo prazo, a dose recomendada é de 40 a 50 UI de ADYNOVI por kg de peso corporal duas vezes por semana a intervalos de 3 a 4 dias. Os ajustes de dose e intervalos de administração podem ser considerados com base nos níveis de fator VIII obtidos e na tendência hemorrágica individual.
População pediátrica
A posologia do tratamento a demanda nos pacientes pediátricos (12 a 18 anos de idade) é a mesma que para os pacientes adultos. O tratamento profilático para pacientes de 12 a <18 anos é o mesmo que para os pacientes adultos. A segurança a longo prazo de ADYNOVI em crianças menores de 12 anos ainda não foi estabelecida. Os ajustes de dose e intervalos de administração podem ser considerados com base nos níveis de fator VIII obtidos e na tendência hemorrágica individual.
- País de registo
- Substância ativa
- Requer receita médicaSim
- Fabricante
- Esta informação é apenas para referência e não constitui aconselhamento médico. Consulte sempre um médico antes de tomar qualquer medicamento. A Oladoctor não se responsabiliza por decisões médicas baseadas neste conteúdo.
- Alternativas a ADYNOVI 3000 UI/5 mL PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVELForma farmacêutica: INJETÁVEL, 1.000 UISubstância ativa: coagulation factor VIIIFabricante: Takeda Manufacturing Austria AgRequer receita médicaForma farmacêutica: INJETÁVEL, 1500 UISubstância ativa: coagulation factor VIIIFabricante: Takeda Manufacturing Austria AgRequer receita médicaForma farmacêutica: INJETÁVEL, 1000 UI - após reconstituição em 2 ml de água para injetáveis, a dose é de 500 UI/mlSubstância ativa: coagulation factor VIIIFabricante: Takeda Manufacturing Austria AgRequer receita médica
Alternativas a ADYNOVI 3000 UI/5 mL PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL noutros países
As melhores alternativas com o mesmo princípio ativo e efeito terapêutico.
Alternativa a ADYNOVI 3000 UI/5 mL PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL em Polónia
Alternativa a ADYNOVI 3000 UI/5 mL PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL em Ukraine
Médicos online para ADYNOVI 3000 UI/5 mL PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL
Avaliação de posologia, efeitos secundários, interações, contraindicações e renovação da receita de ADYNOVI 3000 UI/5 mL PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL – sujeita a avaliação médica e regras locais.
















