
FULVESTRANT STADA 250 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE

How to use FULVESTRANT STADA 250 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Fulvestrant Stada 250 mg solution for injection in pre-filled syringe EFG
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Fulvestrant Stada and what is it used for
- What you need to know before you use Fulvestrant Stada
- How to use Fulvestrant Stada
- Possible side effects
- Storage of Fulvestrant Stada
- Contents of the pack and other information
1. What is Fulvestrant Stada and what is it used for
Fulvestrant Stada contains the active substance fulvestrant, which belongs to the group of estrogen blockers.
Estrogens, a type of female sex hormone, may be involved in the development of breast cancer in some cases.
Fulvestrant is used:
- alone, to treat postmenopausal women with a type of breast cancer called hormone receptor-positive breast cancer, which is locally advanced or has spread to other parts of the body (metastatic), or
- in combination with palbociclib to treat women with a type of breast cancer called hormone receptor-positive, human epidermal growth factor receptor 2 (HER2)-negative breast cancer, which is locally advanced or has spread to other parts of the body (metastatic). Women who have not reached menopause will also be treated with a medicine called a luteinizing hormone-releasing hormone (LHRH) agonist.
Fulvestrant may be administered in combination with palbociclib. It is important that you also read the package leaflet for palbociclib. If you have any questions about palbociclib, ask your doctor.
2. What you need to know before you use Fulvestrant Stada
Do not use Fulvestrant Stada
- if you are allergic to fulvestrant or any of the other ingredients of this medicine (listed in section 6)
- if you are pregnant or breastfeeding
- if you have severe liver problems.
Warnings and precautions
Tell your doctor, pharmacist, or nurse before starting fulvestrant if any of the following apply to you:
- kidney or liver problems
- low platelet count (which helps blood to clot) or bleeding disorders
- previous blood clots
- osteoporosis (loss of bone density)
- alcoholism.
Children and adolescents
Fulvestrant is not indicated in children and adolescents under 18 years of age.
Using Fulvestrant Stada with other medicines
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
In particular, you should tell your doctor if you are using anticoagulants (medicines to prevent blood clots).
Pregnancy and breastfeeding
Pregnancy
Do not use fulvestrant if you are pregnant. If you can become pregnant, you must use an effective contraceptive method while being treated with fulvestrant and for 2 years after your last dose.
Breastfeeding
Do not breastfeed while being treated with fulvestrant.
Driving and using machines
Fulvestrant Stada is not expected to affect your ability to drive or use machines. However, if you feel tired after treatment, do not drive or use machines.
Use in athletes
Patients should be warned that this medicine contains fulvestrant, which may produce a positive result in doping tests.
Fulvestrant Stada contains ethanol
This medicine contains 1,000 mg of alcohol (ethanol) in each 500 mg dose of fulvestrant. The amount of alcohol in each 500 mg dose of this medicine is equivalent to 25 ml of beer or 10 ml of wine.
The amount of alcohol in this medicine is unlikely to have an effect in adults.
The alcohol present in this medicine may alter the effects of other medicines. Talk to your doctor or pharmacist if you are taking other medicines.
If you are addicted to alcohol, talk to your doctor or pharmacist before taking this medicine.
Fulvestrant Stada contains benzyl alcohol
This medicine contains 1,000 mg of benzyl alcohol in each 500 mg dose of fulvestrant. Benzyl alcohol may cause allergic reactions.
Talk to your doctor or pharmacist if you have liver or kidney disease. This is because large amounts of benzyl alcohol may accumulate in the body and cause side effects (metabolic acidosis).
Fulvestrant Stada contains benzyl benzoate
This medicine contains 1,500 mg of benzyl benzoate in each 500 mg dose of fulvestrant.
3. How to use Fulvestrant Stada
Follow exactly the instructions for administration of this medicine given by your doctor or pharmacist. If you are unsure, ask your doctor or pharmacist again.
The recommended dose is 500 mg of fulvestrant (two 250 mg/5 ml injections) administered once a month with an additional dose of 500 mg administered 2 weeks after the initial dose.
Your doctor or nurse will administer fulvestrant by slow intramuscular injection into each of your buttocks.
If you have any other questions about the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, fulvestrant can cause side effects, although not everybody gets them.
You may need urgent medical attention if you experience any of the following side effects:
? Allergic reactions (hypersensitivity), including swelling of the face, lips, tongue, and/or throat, which may be symptoms of anaphylactic reactions.
? Blood clots (thromboembolism)*
? Liver inflammation (hepatitis)
? Liver failure
Tell your doctor, pharmacist, or nurse immediately if you notice any of the following side effects:
Very common side effects(may affect more than 1 in 10 people)
- injection site reactions, such as pain and/or inflammation
- abnormal liver enzyme levels (in blood tests)*
- nausea (feeling sick)
- weakness, fatigue*
- joint and musculoskeletal pain
- hot flashes
- skin rash.
- allergic reactions (hypersensitivity), including swelling of the face, lips, tongue, and/or throat
All other side effects:
Common side effects(may affect up to 1 in 10 people)
- headache
- vomiting, diarrhea, or loss of appetite*
- urinary tract infections
- back pain*
- increased bilirubin (a bile pigment produced by the liver)
- blood clots (thromboembolism)*
- low platelet count (thrombocytopenia)
- vaginal bleeding
- lower back pain radiating to one leg (sciatica)
- sudden weakness, numbness, tingling, or loss of movement in your leg, especially on one side of the body, sudden problems with walking or balance (peripheral neuropathy).
Uncommon side effects(may affect up to 1 in 100 people)
- thick, white, vaginal discharge and candidiasis (infection)
- hematoma and bleeding at the injection site
- increased gamma-GT, a liver enzyme identified in a blood test
- liver inflammation (hepatitis)
- liver failure
- numbness, tingling, and pain
- anaphylactic reactions
- Includes side effects for which the exact role of fulvestrant cannot be assessed due to the underlying disease.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if it is possible that they are not listed in this leaflet. You can also report side effects directly through the Spanish Medicines and Healthcare Products Agency (AEMPS) website: www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Fulvestrant Stada
Keep this medicine out of the sight and reach of children.
Store and transport refrigerated (between 2°C and 8°C).
Keep the pre-filled syringe in the original packaging to protect it from light.
Temperature deviations outside the range of 2°C to 8°C should be controlled. This includes avoiding storage at temperatures above 30°C, and not exceeding a period of 28 days, during which the average storage temperature of the medicine is below 25°C (but above 2°C to 8°C). After temperature deviations, the medicine should be returned immediately to the recommended storage conditions (store and transport in a refrigerator between 2°C and 8°C). Temperature deviations have a cumulative effect on the quality of the medicine, and the 28-day period should not be exceeded beyond the expiration date of fulvestrant. Exposure to temperatures below 2°C will not damage the medicine, as long as it is not stored below -20°C.
Do not use this medicine after the expiry date which is stated on the carton or on the labels of the syringes after the abbreviation EXP. The expiry date is the last day of the month shown.
Your healthcare professional will be responsible for the proper storage, use, and disposal of Fulvestrant Stada.
This medicine may pose a risk to the aquatic environment. Medicines should not be disposed of via wastewater or household waste. Dispose of the packaging and any unused medicine in the pharmacy's SIGRE collection point. Ask your pharmacist how to dispose of the packaging and any unused medicine. This will help protect the environment.
6. Contents of the pack and other information
Composition of Fulvestrant Stada
- The active substance is fulvestrant. Each pre-filled syringe (5 ml) contains 250 mg of fulvestrant. Each ml of solution contains 50 mg of fulvestrant.
- The other ingredients (excipients) are ethanol (96%), benzyl alcohol, benzyl benzoate, and refined castor oil.
Appearance and packaging
Fulvestrant Stada is a clear, colorless to yellow, practically particle-free, oily, and viscous solution, contained in a pre-filled syringe made of glass. Each syringe contains 5 ml of injectable solution.
Fulvestrant Stada is available in three pack sizes:
- Carton box with one blister containing one pre-filled syringe, one sterile hypodermic needle (BD SafetyGlide), and a package leaflet.
or
- Carton box with two blisters, each containing one pre-filled syringe, two sterile hypodermic needles (BD SafetyGlide), and a package leaflet.
or
- Carton box with six blisters, each containing one pre-filled syringe, six sterile hypodermic needles (BD SafetyGlide), and a package leaflet.
Not all pack sizes may be marketed.
Marketing authorization holder
Laboratorio STADA, S.L.
Frederic Mompou, 5
08960 Sant Just Desvern (Barcelona)
Spain
Manufacturer
S.C. Rompharm Company S.R.L.
Strada Eroilor nr. 1A,
Otopeni 075100
Romania
or
STADA Arzneimittel AG
Stadastr. 2-18, Dortelweil
D-61118 Bad Vilbel
Hessen
Germany
or
STADApharm GmbH
Feodor-Lynen-Str. 35
D-30625 Hannover
Germany
or
STADA Arzneimittel GmbH
Geschäftsanschrift -
Muthgasse 36/2 Doebling
A-1190 Vienna
Austria
Date of last revision of this leaflet:December 2020
Detailed information on this medicine is available on the website of the Spanish Agency for Medicines and Healthcare Products (AEMPS) http://www.aemps.gob.es/.
-----------------------------------------------------------------------------------------------
This information is intended only for healthcare professionals:
Fulvestrant Stada should be administered using two pre-filled syringes, see section 3.
Administration instructions
Administer the injection according to local guidelines for intramuscular injection of large volumes.
NOTE: Due to the proximity of the sciatic nerve, caution should be exercised when injecting fulvestrant into the dorsogluteal area (see section 4.4).
Warnings: Do not sterilize the safety needle (BD SafetyGlide) in an autoclave before use.
Hands should remain behind the needle at all times during use and disposal.
For each of the two syringes:
- Remove the glass body of the syringe from the tray (blister) and check that it is not damaged.
- Open the outer package of the safety needle (SafetyGlide).
- Before administration, parenteral solutions should be inspected visually for particulate matter and discoloration.
- Hold the syringe in a vertical position.
- With the other hand, hold the cap and carefully twist and remove it. To maintain sterility, do not touch the tip of the syringe (see Figure 1).
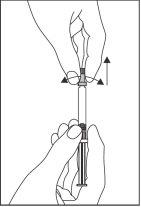
Figure 1
?? Attach the safety needle to the Luer-Lok connector and screw it on until it is firmly attached (see Figure 2).
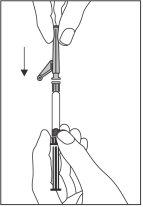
Figure 2
- Check that the needle is attached to the Luer-Lock connector before releasing it from the vertical position.
- Pull the needle protector straight off to avoid damaging the needle tip.
- Take the loaded syringe to the injection site.
- Remove the needle protector.
- Eliminate excess gas from the syringe.
- Administer slowly by intramuscular injection into the buttock (gluteal area) (1-2 minutes/injection). For greater comfort, the position of the needle with the bevel up has the same orientation as the lever raised (see Figure 3).
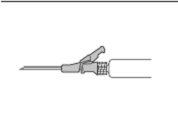
Figure 3
?? After injection, immediately touch the lever with your finger to activate the safety mechanism (see Figure 4).
NOTE: Activate away from your body and others. Listen for the click and visually confirm that the needle tip is fully protected.
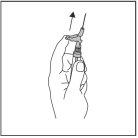
Figure 4
Disposal
The pre-filled syringes are for single use only.
This medicine may pose a risk to the aquatic environment. Disposal of unused medicine and all materials that have come into contact with it will be carried out in accordance with local regulations.
- Country of registration
- Average pharmacy price225.7 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to FULVESTRANT STADA 250 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGEDosage form: INJECTABLE, 250 mg/5 mlActive substance: fulvestrantManufacturer: Bexal Farmaceutica S.A.Prescription requiredDosage form: INJECTABLE, 250 mgActive substance: fulvestrantManufacturer: Ever Valinject GmbhPrescription requiredDosage form: INJECTABLE, 250 mgActive substance: fulvestrantManufacturer: Astrazeneca AbPrescription required
Online doctors for FULVESTRANT STADA 250 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE
Discuss questions about FULVESTRANT STADA 250 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions













