
SUBOXONE 2 mg/0.5 mg SUBLINGUAL FILM-COATED TABLETS

How to use SUBOXONE 2 mg/0.5 mg SUBLINGUAL FILM-COATED TABLETS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Suboxone 2 mg/0.5 mg sublingual film
Suboxone 4 mg/1 mg sublingual film
Suboxone 8 mg/2 mg sublingual film
Suboxone 12 mg/3 mg sublingual film
buprenorphine/naloxone
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Suboxone and what is it used for
- What you need to know before you take Suboxone
- How to take Suboxone
- Possible side effects
- Storing Suboxone
- Contents of the pack and other information
1. What is Suboxone and what is it used for
Suboxone is used to treat opioid dependence (addiction to narcotic drugs such as heroin or morphine) in adults and adolescents who have agreed to be treated for their addiction.
Suboxone is used in adults and adolescents over 15 years of agewho are also receiving medical, social, and psychological support.
2. What you need to know before you take Suboxone
Do not take Suboxone
- if you are allergicto buprenorphine, naloxoneor any of the other ingredients of this medicine (listed in section 6);
- if you have severe respiratory problems;
- if you have severe liver problems;
- if you are suffering from alcohol intoxicationor if you have tremors, sweating, anxiety, confusion, or hallucinations caused by alcohol;
- if you are taking naltrexoneor nalmefenefor the treatment of alcohol or opioid dependence.
Warnings and precautions
Tell your doctor before you start taking Suboxone if you have:
- asthma or other respiratory problems;
- liver problems such as hepatitis;
- low blood pressure;
- a recent head injury or brain disease;
- a urinary disorder (especially associated with an enlarged prostate in men);
- kidney disease;
- thyroid problems;
- a disorder of the adrenal gland (e.g., Addison's disease);
- depression or other illnesses that are treated with antidepressants. The use of these medicines with Suboxone may cause serotonin syndrome, a potentially life-threatening disease (see "Other medicines and Suboxone").
Important things to remember:
- In case of accidental ingestion or suspected ingestion, contact an emergency unit immediately.
- Additional monitoring
If you are over 65 years old, your doctor may monitor you more closely.
- Misuse and abuse
This medicine may be targeted by people who abuse prescription medicines and should be kept in a safe place to protect it from theft (see section 5). Do not give this medicine to anyone else. It may cause them to die or have other serious problems.
- Respiratory problems
Some people have died from respiratory failure (inability to breathe) because they used buprenorphine improperly or took it with other central nervous system depressants, such as alcohol, benzodiazepines (tranquilizers), or other opioids.
This medicine can cause severe respiratory depression (difficulty breathing) in children and non-dependent persons if they take it accidentally or intentionally.
- Dependence
This medicine can cause dependence.
- Withdrawal symptoms
This medicine can cause opioid withdrawal symptoms if you take it too soon after using opioids. You should wait at least 6 hours after using a short-acting opioid (e.g., morphine, heroin) or at least 24 hours after using a long-acting opioid, such as methadone.
This medicine can also cause withdrawal symptoms if you stop taking it suddenly. See section 3 "If you stop treatment".
- Liver damage
There have been reports of liver damage after taking Suboxone, especially when the medicine is misused. It may also be due to viral infections (e.g., chronic hepatitis C), alcohol abuse, anorexia, or the use of other medicines that can damage the liver (see section 4). Your doctor may perform frequent blood tests to monitor your liver function. Tell your doctor if you have any liver problems before starting treatment with Suboxone.
- Blood pressure
This medicine can cause a sudden drop in blood pressure, making you feel dizzy if you get up too quickly after sitting or lying down.
- Diagnosis of unrelated medical conditions
This medicine can mask the symptoms of pain that could help in the diagnosis of some diseases. You should tell your doctor that you are taking this medicine.
Children and adolescents
Do not give this medicine to children under 15 years of age. If you are between 15 and 18 years old, your doctor may monitor you more closely during treatment due to the lack of data in this age group.
Other medicines and Suboxone
Tell your doctor if you are taking, have recently taken, or might take any other medicines.
Some medicines can increase the adverse effects of Suboxone and may be serious. Do not take other medicines at the same time as Suboxone without consulting your doctor first, especially:
- Benzodiazepines(used to treat anxiety or sleep disorders) such as diazepam, temazepam, or alprazolam. The concomitant use of Suboxone and sedatives like benzodiazepines or related medicines increases the risk of drowsiness, difficulty breathing (respiratory depression), and coma, and may be potentially fatal. For this reason, concomitant use should only be considered when other treatment options are not possible.
However, if your doctor prescribes Suboxone with sedatives, your doctor should limit the dose and duration of concomitant treatment.
Tell your doctor about all sedatives you are taking and strictly follow your doctor's dosage recommendation. It may be helpful to inform your friends or family to be alert to the signs and symptoms mentioned above. Contact your doctor if you experience these symptoms.
- Other medicines that can cause drowsiness and are used to treat diseases such as anxiety, insomnia, seizures/epileptic fits, or pain. These types of medicines can reduce your level of alertness, making it difficult to drive and use machines. They can also cause central nervous system depression, which is very serious. The following is a list of examples of these types of medicines:
- other medicines that contain opioids, such as methadone, some painkillers, or cough suppressants;
- antidepressants (used to treat depression) such as isocarboxazid, phenelzine, selegiline, tranylcypromine, and valproate may enhance the effects of this medicine;
- sedating H1 receptor antagonists (used to treat allergic reactions) such as diphenhydramine and chlorphenamine;
- barbiturates (used to produce sleep or sedation) such as phenobarbital or secobarbital;
- tranquilizers (used to produce sleep or sedation) such as chloral hydrate.
- Antidepressantssuch as moclobemide, tranylcypromine, citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, sertraline, duloxetine, venlafaxine, amitriptyline, doxepin, or trimipramine. These medicines can interact with Suboxone, and you may experience symptoms such as involuntary muscle contractions, including muscles that control eye movement, agitation, hallucinations, coma, excessive sweating, tremors, exaggerated reflexes, increased muscle tension, body temperature above 38 °C. Contact your doctor if you experience these symptoms.
- The clonidine (used to treat high blood pressure) may prolong the effects of this medicine.
- Antiretrovirals (used to treat HIV) such as ritonavir, nelfinavir, or indinavir may enhance the effects of this medicine.
- Certain antifungals (used to treat fungal infections) such as ketoconazole, itraconazole, or certain antibiotics may prolong the effects of this medicine.
- Some medicines can reduce the effect of Suboxone. These include medicines used to treat epilepsy (carbamazepine and phenytoin) and medicines used to treat tuberculosis (rifampicin).
- Naltrexone and nalmefene (medicines used to treat addictive disorders) may block the therapeutic effects of Suboxone. They should not be taken at the same time as Suboxone treatment, as you may experience a sudden onset of prolonged and intense withdrawal.
Using Suboxone with food, drinks, and alcohol
Do not drink alcoholwhile you are being treated with this medicine. Alcohol may increase drowsiness and the risk of respiratory failure if taken with Suboxone. Do not swallow or consume food or drinks until the film has dissolved completely.
Pregnancy, breastfeeding, and fertility
Tell your doctor if you are pregnant, think you may be pregnant, or plan to become pregnant. The risks of using Suboxone in pregnant women are not known. Your doctor will decide whether you should continue your treatment with another medicine.
When taken during pregnancy, especially in the last months, medicines like Suboxone can cause withdrawal symptoms in the newborn, including respiratory problems. This can occur several days after birth.
Do not breastfeed while taking this medicine, as buprenorphine is excreted in breast milk.
Ask your doctor or pharmacist for advice before taking any medicine.
Driving and using machines
Do not drive or ride a bike, do not use tools or machines, or engage in hazardous activities until you know how this medicine affects you. Suboxone can cause drowsiness, dizziness, or altered thinking. This can happen more often in the first few weeks of treatment, when the dose is being changed, but it can also happen if you drink alcohol or take other sedative medicines at the same time as you take Suboxone.
Suboxone contains maltitol, orange yellow S (E-110), and sodium.
Suboxone contains liquid maltitol. If your doctor has told you that you have an intolerance to some sugars, consult them before taking this medicine.
Suboxone contains orange yellow S (E-110), which may cause allergic reactions.
This medicine contains less than 1 mmol of sodium (23 mg) per film; i.e., it is essentially "sodium-free".
3. How to Take Suboxone
Follow your doctor's or pharmacist's instructions for taking this medication exactly. If you are unsure, consult your doctor or pharmacist again.
Treatment is prescribed and supervised by doctors with experience in treating drug addiction.
Your doctor will determine the best dose for you. During treatment, your doctor may adjust your dose based on your response to treatment.
Starting Treatment
The recommended initial dose in adults and adolescents over 15 years is normally two Suboxone 2 mg/0.5 mg sublingual films or one Suboxone 4 mg/1 mg sublingual film.
This dose may be repeated two more times on Day 1, depending on your needs.
Before taking your first dose of Suboxone, you must be aware of clear withdrawal symptoms. Your doctor will tell you when to take your first dose.
- Starting treatment with Suboxone if you have heroin dependence
If you have heroin or short-acting opioid dependence, the first dose should be taken when withdrawal signs appear, at least 6 hours after last opioid use.
- Starting treatment with Suboxone if you have methadone dependence
If you have been taking methadone or long-acting opioids, it is recommended to reduce the methadone dose to less than 30 mg/day before starting Suboxone treatment. The first Suboxone dose should be taken when withdrawal signs appear and at least 24 hours after last methadone use.
Dose Adjustment and Maintenance Treatment:During the days following the start of treatment, your doctor may increase your Suboxone dose as needed. If you feel the effect of Suboxone is too strong or too weak, inform your doctor or pharmacist. The maximum daily dose is 24 mg of buprenorphine.
After a period of satisfactory treatment, you may agree with your doctor to gradually decrease the dose to a lower maintenance dose.
How to Take Suboxone
- Take the dose once a day, approximately at the same time each day.
- It is recommended to moisten your mouth before taking the film.
- Place the sublingual film under your tongue (sublingually) or inside your cheek (buccally) as directed by your doctor. Make sure the films do not overlap.
- Keep the films under your tongue or inside your cheek until they have completely dissolved.
- Do not chew or swallowthe film, as the medication will not work and you may experience withdrawal symptoms.
- Do not consume food or drinks until the film has completely dissolved.
- Do not split or cut the film into smaller doses.
How to Remove the Film from the Pouch
Each Suboxone film comes in a child-resistant pouch. Do not open the pouch until you are ready to use it.
To open the pouch, locate the dotted line on the top edge of the pouch and fold the pouch edge along that line (see Figure 1).
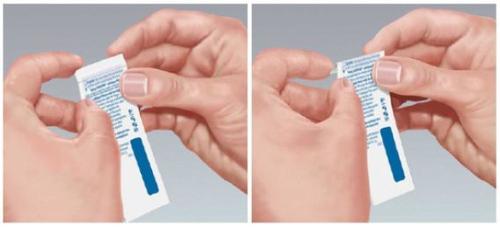
Figure 1
- When folding the pouch along the dotted line, a slit appears on the folded edge of the pouch that can be torn along the arrow.
- Alternatively, the pouch can be cut with scissors along the arrow (see Figure 2).
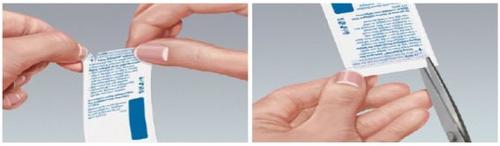
Figure 2
If the pouch is damaged, discard the film.
How to Place a Film Under Your Tongue (Sublingually):
First, drink water to moisten your mouth. This helps the film dissolve more easily. Then, hold the film between two fingers by the outer edges and place it under your tongue, near the base, either on the right or left side (see Figure 3).
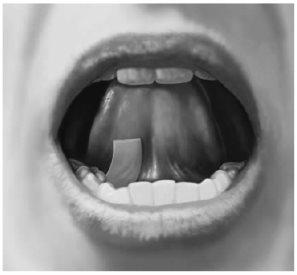
Figure 3
If your doctor has instructed you to take two films at the same time, place the second film under your tongue on the other side. Make sure the films do not overlap.
If your doctor has instructed you to take a third film, place it under your tongue on either side after the first two have dissolved.
How to Place a Film Inside Your Cheek (Buccally):
Drink water to moisten your mouth. Hold the film between two fingers by the outer edges and place it inside your cheek, either on the right or left side (see Figure 4).
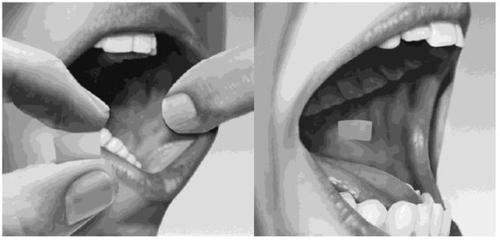
Figure 4
If your doctor has instructed you to take two films at the same time, place the second film inside the other cheek; this will ensure the films do not overlap. If your doctor has instructed you to take a third film, place it inside your cheek on either side after the first two have dissolved.
If You Take More Suboxone Than You Should
If you or someone else takes too much of this medication, seek urgent medical attention.
Suboxone overdose can cause serious and potentially life-threatening breathing problems.
Overdose symptoms may include drowsiness and discoordination with slow reflexes, blurred vision, and/or difficulty speaking. You may not be able to think clearly and may breathe much more slowly than normal for you.
If You Miss a Dose of Suboxone
If you forget to take a dose, inform your doctor as soon as possible.
If You Stop Treatment with Suboxone
Sudden interruption of treatment can cause withdrawal symptoms.Depending on your condition, the Suboxone dose may continue to be decreased under close medical supervision until it can finally be stopped. Do not change your treatment in any way or stop it without your doctor's authorization.
If you have any other questions about using this medication, ask your doctor or pharmacist.
4. Possible Side Effects
Like all medications, this medication can cause side effects, although not everyone may experience them.
Tell your doctor or seek urgent medical attention immediatelyif you experience side effects such as:
- Swelling of the face, lips, tongue, or throat, which can cause difficulty swallowing or breathing; severe hives/urticaria. These can be signs of a potentially life-threatening allergic reaction.
- Drowsiness and discoordination, blurred vision, difficulty speaking, inability to think clearly, or breathing much more slowly than normal for you.
- Extreme fatigue, itching with yellowing of the skin or eyes. These can be signs of liver damage.
- Seeing or hearing things that do not really exist (hallucinations).
Very Common Side Effects (may affect more than 1 in 10 people):
- Insomnia (inability to sleep),
- constipation,
- nausea,
- excessive sweating,
- headache,
- drug withdrawal syndrome.
Common Side Effects (may affect up to 1 in 10 people):
- weight loss,
- swelling of hands and feet,
- drowsiness,
- anxiety,
- nervousness,
- tingling,
- depression,
- decreased sexual desire,
- increased muscle tension,
- abnormal thinking,
- increased tear production or other tear disorders,
- hot flashes,
- increased blood pressure,
- migraines,
- runny nose,
- throat pain and difficulty swallowing,
- increased coughing,
- stomach upset or other stomach discomfort,
- diarrhea,
- mouth redness,
- abnormal liver function,
- flatulence,
- vomiting,
- skin rash,
- itching,
- hives,
- pain,
- joint pain,
- muscle pain,
- leg cramps (muscle spasms),
- difficulty getting or maintaining an erection,
- urine abnormalities,
- abdominal pain,
- back pain,
- weakness,
- infection,
- chills,
- chest pain,
- fever,
- flu-like symptoms,
- general feeling of discomfort,
- accidental injury due to loss of alertness or coordination,
- fainting,
- dizziness.
Uncommon Side Effects (may affect up to 1 in 100 people):
- swollen glands (lymph nodes),
- agitation,
- tremor,
- abnormal dreams,
- excessive muscle activity,
- depersonalization (feeling not like oneself),
- drug dependence,
- amnesia (memory disorder),
- loss of interest,
- attention disorder,
- exaggerated feeling of well-being,
- seizure (epileptic fits),
- speech disorder,
- small pupils,
- difficulty urinating,
- blurred vision,
- eye inflammation or infection,
- fast or slow heartbeat,
- low blood pressure,
- palpitations,
- heart attack,
- chest tightness,
- shortness of breath,
- asthma,
- yawning,
- mouth problems (sores, blisters, numbness, tingling, swelling, or pain),
- tongue discoloration or pain,
- acne,
- skin nodule,
- hair loss,
- dry or flaky skin,
- joint inflammation,
- urinary tract infection,
- abnormal blood tests,
- blood in the urine,
- abnormal ejaculation,
- menstrual or vaginal problems,
- kidney stone,
- protein in the urine,
- pain or difficulty urinating,
- sensitivity to heat or cold,
- heat stroke,
- allergic reaction,
- loss of appetite,
- hostile feelings,
- poisoning.
Frequency Not Known (cannot be estimated from the available data):
- sudden withdrawal syndrome caused by taking Suboxone too soon after illegal opioid use,
- drug withdrawal syndrome in newborns,
- slow or difficult breathing,
- liver damage with or without jaundice,
- hallucinations,
- swelling of the face and throat or potentially life-threatening allergic reactions,
- drop in blood pressure when changing position from sitting or lying down to standing, causing dizziness,
- irritation or inflammation of the mouth, including under the tongue.
Misuse of this medication by injection can cause withdrawal symptoms, infections, other skin reactions, and potentially serious liver problems (see "Warnings and Precautions").
Reporting Side Effects
If you experience any side effects, consult your doctor or pharmacist, even if they are not listed in this leaflet. You can also report them directly through the national reporting system included in Appendix V. By reporting side effects, you can help provide more information on the safety of this medication.
5. Storage of Suboxone
Keep this medication out of sight and reach of children and other family members. Do not use this medication after the expiration date shown on the carton and pouch. The expiration date is the last day of the month indicated.
Store below 25°C.
Suboxone may be a target for people who abuse prescription medications.
Keep this medication in a safe place to protect it from theft.
Store the pouch safely.
Never open the pouch before it is time.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of the packaging and any unused medication. This will help protect the environment.
6. Package Contents and Additional Information
Suboxone Composition
- The active ingredients are buprenorphine and naloxone.
Each 2 mg/0.5 mg film contains 2 mg of buprenorphine (as hydrochloride) and 0.5 mg of naloxone (as hydrochloride dihydrate).
Each 4 mg/1 mg film contains 4 mg of buprenorphine (as hydrochloride) and 1 mg of naloxone (as hydrochloride dihydrate).
Each 8 mg/2 mg film contains 8 mg of buprenorphine (as hydrochloride) and 2 mg of naloxone (as hydrochloride dihydrate).
Each 12 mg/3 mg film contains 12 mg of buprenorphine (as hydrochloride) and 3 mg of naloxone (as hydrochloride dihydrate).
- The other ingredients are macrogol, liquid maltitol, natural lime flavor, hypromellose, citric acid, potassium acesulfame, sodium citrate, orange yellow S (E-110), and white ink.
Product Appearance and Package Contents
Suboxone 2 mg/0.5 mg sublingual films are orange rectangular films with nominal dimensions of 22.0 mm × 12.8 mm, with “N2” engraved in white ink.
Suboxone 4 mg/1 mg sublingual films are orange rectangular films with nominal dimensions of 22.0 mm × 25.6 mm, with “N4” engraved in white ink.
Suboxone 8 mg/2 mg sublingual films are orange rectangular films with nominal dimensions of 22.0 mm × 12.8 mm, with “N8” engraved in white ink.
Suboxone 12 mg/3 mg sublingual films are orange rectangular films with nominal dimensions of 22.0 mm × 19.2 mm, with “N12” engraved in white ink.
The films are packaged in individual pouches.
Package sizes: boxes containing 7 × 1, 14 × 1, and 28 × 1 films.
Only some package sizes may be marketed.
Marketing Authorization Holder
Indivior Europe Limited
27 Windsor Place
Dublin 2
Ireland
Manufacturer
Almac Pharma Services Limited
Seagoe Industrial Estate, Portadown
Craigavon BT63 5UA
United Kingdom
Almac Pharma Services (Ireland) Limited
Finnabair Industrial Estate, Dundalk
Co. Louth A91 P9KD
Ireland
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
Belgium Indivior Europe Limited Tel: 0800 780 41 e-mail: [email protected] | Lithuania Indivior Europe Limited Tel: 880030793 e-mail: [email protected] |
Greece Indivior Europe Limited Tel: 00800 110 4104 e-mail: [email protected] | Luxembourg Indivior Europe Limited Tel: 800 245 43 e-mail: [email protected] |
Czech Republic Indivior Europe Limited Tel: 800 143 737 e-mail: [email protected] | Hungary Indivior Europe Limited Tel: 6800 19301 e-mail: [email protected] |
Denmark Indivior Europe Limited Tlf: 80826653 e-mail: [email protected] | Malta Indivior Europe Limited Tel: 80062185 e-mail: [email protected] |
Germany Indivior Europe Limited Tel: 800 181 3799 e-mail: [email protected] | Netherlands Indivior Europe Limited Tel: 0800 022 87 83 e-mail: [email protected] |
Estonia Indivior Europe Limited Tel: 8000041004 e-mail: [email protected] | Norway Indivior Europe Limited Tlf: 80016773 e-mail: [email protected] |
Greece Indivior Europe Limited Tel: 800 270 81 901 e-mail: [email protected] | Austria Indivior Europe Limited Tel: 800 296551 e-mail: [email protected] |
Spain Indivior Europe Limited Tel: 900 994 121 e-mail: [email protected] | Poland Indivior Europe Limited Tel: 0800 4111237 e-mail: [email protected] |
France Indivior Europe Limited Tel: 0800 909 972 e-mail: [email protected] | Portugal Indivior Europe Limited Tel: 800 841 042 e-mail: [email protected] |
Croatia Indivior Europe Limited Tel: + 0800 222 899 e-mail: [email protected] | Romania Indivior Europe Limited Tel: 800 477 029 e-mail: [email protected] |
Ireland Indivior Europe Limited Tel: 1800554156 e-mail: [email protected] | Slovenia Indivior Europe Limited Tel: 080080715 e-mail: [email protected] |
Iceland Indivior Europe Limited Phone: 8009875 e-mail: [email protected] | Slovakia Indivior Europe Limited Tel: 800110286 e-mail: [email protected] |
Italy Indivior Europe Limited Tel: 800 789 822 e-mail: [email protected] | Finland Indivior Europe Limited Phone/Tel: 0800417489 e-mail: [email protected] |
Cyprus Indivior Europe Limited Tel: 800 270 81 901 e-mail: [email protected] | Sweden Indivior Europe Limited Tel: 020791680 e-mail: [email protected] |
Latvia Indivior Europe Limited Tel: 800 05612 e-mail: [email protected] | United Kingdom Indivior Europe Limited Tel: 0808 234 9243 e-mail: [email protected] |
Date of Last Revision of this Leaflet:{month YYYY}.
Detailed information on this medication is available on the European Medicines Agency website: http://www.ema.europa.eu/.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to SUBOXONE 2 mg/0.5 mg SUBLINGUAL FILM-COATED TABLETSDosage form: SUBLINGUAL TABLET, 2 mg/0.5 mgActive substance: buprenorphine, combinationsManufacturer: Aurovitas Spain, S.A.U.Prescription requiredDosage form: SUBLINGUAL TABLET, 8 mg/2 mgActive substance: buprenorphine, combinationsManufacturer: Aurovitas Spain, S.A.U.Prescription requiredDosage form: SUBLINGUAL TABLET, 12 mg/3 mgActive substance: buprenorphine, combinationsManufacturer: Indivior Europe LimitedPrescription required
Online doctors for SUBOXONE 2 mg/0.5 mg SUBLINGUAL FILM-COATED TABLETS
Discuss questions about SUBOXONE 2 mg/0.5 mg SUBLINGUAL FILM-COATED TABLETS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions













