SONOVUE 8 microliters/ml POWDER AND SOLVENT FOR INJECTABLE DISPERSION

How to use SONOVUE 8 microliters/ml POWDER AND SOLVENT FOR INJECTABLE DISPERSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
SonoVue 8 microliters/ml powder and solvent for injectable dispersion
Hexafluoride sulfur
|
Contents of the package leaflet
- What is SonoVue and what is it used for
- What you need to know before you use SonoVue
- How to use SonoVue
- Possible side effects
- Storage of SonoVue
- Contents of the pack and further information
1. What is SonoVue and what is it used for
SonoVue is indicated only for diagnostic use.
SonoVue is an ultrasound contrast agent that contains microbubbles filled with a gas called sulfur hexafluoride.
If you are an adult, SonoVue helps you get clearer ultrasound images of the heart, blood vessels, and/or liver and breast tissues.
SonoVue helps get clearer images of the urinary tract in children.
2. What you need to know before you use SonoVue
Do not use SonoVue:
- If you are allergic to sulfur hexafluoride or any of the other ingredients of this medicine (listed in section 6).
- If your doctor has told you that you have a right-to-left cardiac shunt.
- If you have severe pulmonary hypertension (pulmonary artery pressure > 90 mm Hg).
- If you have uncontrolled hypertension.
- If you have adult respiratory distress syndrome (a severe disease characterized by widespread inflammation in the lungs).
- If you have been told not to take dobutamine (a heart-stimulating medication) due to severe heart disease.
Warnings and precautions
Tell your doctor if you have had:
- episodes of angina or chest pain (thoracic) frequent and/or repeated, especially if you have a history of heart disease,
- recent changes in your electrocardiogram.
Tell your doctor before you are given SonoVue if:
- you have recently had a myocardial infarction or have recently undergone coronary intervention,
- you have chest pain, angina, or severe heart disease,
- you have severe heart rhythm disorders,
- your heart disease has worsened recently,
- you have acute cardiac inflammation of the heart membrane (endocarditis),
- you have artificial heart valves implanted,
- you have acute generalized inflammation or infection,
- you have known blood coagulation problems,
- you have severe kidney or liver disease,
If you are given SonoVue in combination with a medication, exercise, or device that stimulates the heart to visualize its activity under stress, your heart activity, blood pressure, and heart rate will be monitored.
Children and adolescents
For patients under 18 years of age, SonoVue can only be used for ultrasound of the urinary tract.
Using SonoVue with other medicines
Tell your doctor if you are taking, have recently taken, or might take any other medicines.
In particular, tell your doctor if you are taking beta-blockers (medicines to treat heart disease and hypertension or administered as eye drops to treat glaucoma).
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
It is not known whether SonoVue passes into breast milk. However, breastfeeding should be interrupted for two or three hours after the ultrasound.
Driving and using machines
SonoVue does not affect your ability to drive and use machines.
SonoVue contains sodium
This medicine contains less than 23 mg (1 mmol) of sodium per dose, so it is essentially 'sodium-free'.
3. How to use SonoVue
SonoVue will be administered to you by qualified medical or healthcare personnel.
For ultrasound of the heart or its blood vessels and/or liver and breast tissues in adults: the dose to be administered into a vein will be calculated based on the part of the body to be examined. The recommended dose is 2 or 2.4 ml per patient. This dose may be repeated if necessary up to a maximum of 4.8 ml.
For ultrasound of the urinary tract in children: the recommended dose is 1 ml per patient, to be administered into the bladder as follows:
After emptying your bladder, a saline solution will be introduced into your bladder through a thin tube. Then, SonoVue will be administered through the thin tube, followed by the saline solution to continue filling the bladder. The filling and emptying of the bladder with saline solution may be repeated if necessary.
If you have a serious lung or heart problem, the medical staff will closely monitor you during the administration of SonoVue and for at least 30 minutes after.
If you use more SonoVue than you should
Overdose is unlikely since SonoVue is administered by a healthcare professional. If an overdose occurs, the doctor will take the necessary measures.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Most side effects of SonoVue are rare and not serious. However, some patients may experience serious side effects and may need treatment.
Tell your doctor immediatelyif you notice any of the following side effects,
because you may need medical treatment: swelling of the face, lips, mouth, or throat that may make swallowing or breathing difficult; skin rash; hives; swelling of the hands, feet, or ankles.
The following side effects have been reported with the use of SonoVue:
Uncommon side effects(may affect up to 1 in 100 people):
- Headache,
- Numbness,
- Dizziness,
- Unusual taste in the mouth,
- Redness,
- Chest discomfort,
- Feeling of discomfort (nausea),
- Abdominal pain,
- Skin rash,
- Feeling of heat,
- Local reactions at the injection site, such as: pain or an unusual sensation at the injection site.
Rare side effects(may affect up to 1 in 1,000 people):
- Blurred vision,
- Decreased blood pressure,
- Itching,
- Back pain,
- General pain,
- Chest pain,
- Fatigue,
- Severe and less severe allergic reactions (including skin redness, decreased heart rate, decreased blood pressure, shortness of breath, loss of consciousness, cardiac arrest, or a more severe reaction accompanied by difficulty breathing and dizziness).
Frequency not known(cannot be estimated from the available data):
- Chest pain that extends to the neck or left arm, as it may be a symptom of a potentially severe allergic reaction called Kounis syndrome.
- Fainting,
- In some cases of allergic reactions in patients with angiocardiopathy, cases of lack of oxygen supply to the heart or cardiac arrest have been reported,
- Vomiting.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of SonoVue
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label after EXP. The expiry date is the last day of the month stated.
This medicine does not require any special storage conditions.
The SonoVue dispersion should be administered within 6 hours of preparation.
6. Contents of the pack and further information
Contents of SonoVue
- The active substance is sulfur hexafluoride in the form of microbubbles.
- The other ingredients are: macrogol 4000, distearoylphosphatidylcholine, dipalmitoylphosphatidylglycerol sodium, palmitic acid.
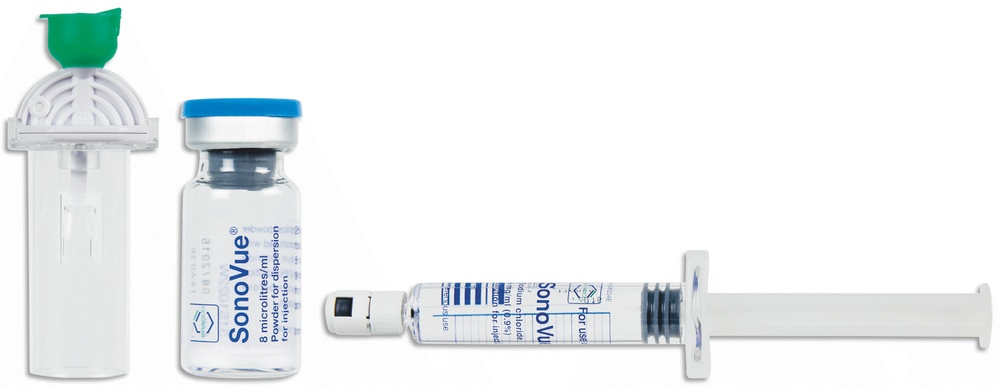
The glass syringe contains a 9 mg/ml (0.9%) sodium chloride injectable solution. Appearance of the product and pack size
SonoVue is a kit that includes a glass vial with white powder, a glass syringe containing the solvent, and a transfer system.
Marketing authorisation holder and manufacturer:
Marketing authorisation holder
Bracco International B.V.
Strawinskylaan 3051
NL-1077 ZX Amsterdam
Netherlands
Manufacturer:
Bracco Imaging S.p.A.
Via Ribes 5, Bioindustry Park
Colleretto Giacosa-10010 (TO)
Italy
Date of last revision of this leaflet:
Other sources of information
Detailed information on this medicine is available on the European Medicines Agency website: http://www.ema.europa.eu.
This information is intended only for healthcare professionals:
If SonoVue is not used immediately after reconstitution, the dispersion will be shaken again before being drawn up into the syringe.
The medicine is for single use only. Any unused volume at the end of an examination should be discarded.
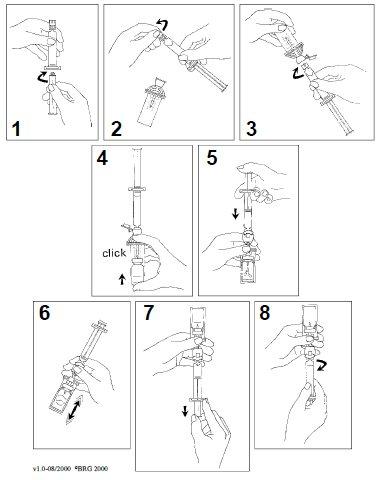
Reconstitution instructions:
|
- Connect the plunger to the syringe by screwing it clockwise.
- Open the blister of the MiniSpike transfer system and remove the cap from the syringe.
- Open the cap of the transfer system and connect the syringe by screwing it clockwise.
- Remove the protective disc from the vial. Slide the vial into the transparent sheet of the transfer system and press firmly to secure the vial in place.
- Empty the contents of the syringe into the vial by pushing the plunger.
- Shake vigorously for 20 seconds to mix the contents of the vial to obtain a homogeneous milky liquid.
- Invert the system and carefully draw up SonoVue into the syringe.
- Unscrew the syringe from the transfer system.
After reconstitution, SonoVue is a homogeneous white milky dispersion.
Do not use if the resulting liquid is clear and/or if solid parts of the lyophilized product are observed in the suspension.
The SonoVue injectable dispersion should be administered within 6 hours of preparation.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to SONOVUE 8 microliters/ml POWDER AND SOLVENT FOR INJECTABLE DISPERSIONDosage form: INJECTABLEActive substance: perflutren, phospholipid microspheresManufacturer: Lantheus Eu LimitedPrescription requiredDosage form: INJECTABLE, 0.19 mg/mlActive substance: perflutren, human albumin microspheresManufacturer: Ge Healthcare AsPrescription requiredDosage form: INJECTABLE, 0.19 mg/mlActive substance: perflutren, human albumin microspheresManufacturer: Ge Healthcare AsPrescription required
Online doctors for SONOVUE 8 microliters/ml POWDER AND SOLVENT FOR INJECTABLE DISPERSION
Discuss questions about SONOVUE 8 microliters/ml POWDER AND SOLVENT FOR INJECTABLE DISPERSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions