
SOMAVERT 20 mg POWDER AND SOLVENT FOR INJECTABLE SOLUTION


How to use SOMAVERT 20 mg POWDER AND SOLVENT FOR INJECTABLE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
SOMAVERT 10 mg powder and solvent for solution for injection
SOMAVERT 15 mg powder and solvent for solution for injection
SOMAVERT 20 mg powder and solvent for solution for injection
SOMAVERT 25mg powder and solvent for solution for injection
SOMAVERT 30mg powder and solvent for solution for injection
pegvisomant
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is SOMAVERT and what is it used for
- What you need to know before you use SOMAVERT
- How to use SOMAVERT
- Possible side effects
- Storage of SOMAVERT
- Contents of the pack and other information
1. What is SOMAVERT and what is it used for
SOMAVERT is used to treat acromegaly, a hormonal disorder resulting from increased secretion of growth hormone (GH) and IGF-I (insulin-like growth factor), and is characterized by overgrowth of bones, thickening of soft tissues, heart disease, and related disorders.
The active substance of SOMAVERT, pegvisomant, is known as a growth hormone receptor antagonist. These substances reduce the action of GH and IGF-I levels circulating in the blood.
2. What you need to know before you use SOMAVERT
Do not use SOMAVERT
- If you are allergic to pegvisomant or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before you start using SOMAVERT.
- If you notice vision disturbances or headaches, you should tell your doctor immediately.
- Your doctor or nurse will check the levels of IGF-I (insulin-like growth factor) circulating in your blood and, if necessary, adjust the dose of SOMAVERT.
- Your doctor should also check your adenoma (benign tumor).
- Your doctor will perform liver function tests before starting and during treatment with SOMAVERT. If the results of these tests are not normal, your doctor will discuss treatment options with you. Once treatment begins, your doctor or nurse will check liver enzyme levels in your blood every 4-6 weeks during the first 6 months of treatment with SOMAVERT. Administration of SOMAVERT should be suspended if symptoms of liver disease persist.
- If you are diabetic, your doctor may need to adjust the amount of insulin or other medications you are using.
- Fertility in female patients may increase as the disease improves. This medicine is not recommended for use in pregnant women, and women of childbearing age should be advised to use a contraceptive method. See also the section on Pregnancy below.
Other medicines and SOMAVERT
Tell your doctor if you have previously used another medicine for the treatment of acromegaly or any medicine for the treatment of diabetes.
Tell your doctor or pharmacist if you are using or have recently used any other medicines. As part of your treatment, you may be treated with other medicines. It is important that you continue to use all medicines, including SOMAVERT, unless your doctor, pharmacist, or nurse tells you otherwise.
Pregnancy, breastfeeding, and fertility
SOMAVERT is not recommended for use in pregnant women. If you are a woman of childbearing age, you should use a contraceptive method during treatment.
It is not known whether pegvisomant passes into breast milk. You should not breastfeed while taking SOMAVERT unless you have discussed it with your doctor.
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, ask your doctor or pharmacist for advice before using this medicine.
Driving and using machines
No studies have been performed on the effects on the ability to drive and use machines.
SOMAVERT contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per dose; this is essentially "sodium-free".
3. How to use SOMAVERT
Follow exactly the administration instructions of this medicine given by your doctor or pharmacist. If you are unsure, ask your doctor or pharmacist again.
Your doctor will administer an initial dose of 80 mg of pegvisomant subcutaneously (under the skin). Then, the usual daily dose is 10 mg of pegvisomant administered by subcutaneous injection (under the skin).
Every 4-6 weeks, your doctor will make the necessary dose adjustments by increasing the dose by 5 mg of pegvisomant per day, according to your blood levels of IGF-I, in order to achieve an optimal therapeutic response.
Form and route of administration
SOMAVERT is injected under the skin. The injection can be given by you or another person, such as your doctor or helper. You should follow the detailed instructions on the injection process, which are included at the end of this leaflet. You should continue to inject this medicine for as long as your doctor tells you.
This medicine must be dissolved before use. The injection must not be mixed in the same syringe or vial with another medicine.
The fatty tissue under the skin may increase at the injection site. To avoid this, use slightly different injection points each time, as described in step 2 of the section of this leaflet "Instructions for preparation and administration of a SOMAVERT injection". This will give your skin and the area under the skin time to recover between injections before injecting again in the same place.
If you feel that the effect of this medicine is too strong or too weak, talk to your doctor, pharmacist, or nurse.
If you inject more SOMAVERT than you should
If you accidentally inject more SOMAVERT than your doctor told you, it is unlikely to be serious, but you should tell your doctor, pharmacist, or nurse immediately.
If you forget to use SOMAVERT
If you forget to give an injection, you should inject the next dose as soon as you remember and continue to inject SOMAVERT as prescribed by your doctor. Do not inject a double dose to make up for forgotten doses.
If you have any other questions about the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Allergic reactions (anaphylactic) from mild to severe have been reported in some patients using SOMAVERT. Symptoms of a severe allergic reaction can include one or more of the following: swelling of the face, tongue, lips, or throat; wheezing or difficulty breathing (laryngeal spasm); widespread skin rash, hives, or itching; or dizziness. Contact your doctor immediately if you experience any of these symptoms.
Very common: may affect more than 1 in 10 people:
- Headache.
- Diarrhea.
- Joint pain.
Common: may affect up to 1 in 10 people:
- Difficulty breathing.
- Increases in liver enzyme levels. These can be seen in blood test results.
- Blood in the urine.
- High blood pressure.
- Constipation, nausea, feeling sick, bloating, indigestion, flatulence.
- Dizziness, drowsiness, tremor, decreased sense of touch.
- Bruising or bleeding at the injection site, pain or swelling at the injection site, increased fatty tissue under the skin at the injection site, swelling of the limbs, weakness, fever.
- Sweating, itching, rash, tendency to bruise.
- Muscle pain, arthritis.
- Increased cholesterol in the blood, weight gain, increased glucose in the blood, decreased glucose in the blood.
- Flu-like symptoms, fatigue.
- Abnormal dreams.
- Eye pain.
Uncommon: may affect up to 1 in 100 people:
- Allergic reaction after administration (fever, rash, itching, and, in severe cases, difficulty breathing, rapid swelling of the skin, requiring urgent medical attention). These can occur immediately or several days after administration.
- Protein in the urine, increased urine output, kidney problems.
- Lack of interest, feeling confused, increased libido, panic attacks, memory loss, sleep disturbances.
- Decreased platelet count in the blood, increased or decreased white blood cell count in the blood, tendency to bleed.
- Abnormal sensation, altered wound healing.
- Eye heaviness, inner ear problems.
- Facial swelling, dry skin, night sweats, skin redness (erythema), itching, and hives.
- Increased fat in the blood, increased appetite.
- Dry mouth, increased salivation, dental problems, hemorrhoids.
- Abnormal taste, migraines.
Rare: cannot be estimated from the available data
- Irritability.
- Severe difficulty breathing (laryngospasm).
- Rapid swelling of the skin, underlying tissue, and mucous membranes (angioedema).
About 17% of patients will develop antibodies to growth hormone during treatment. It seems that these antibodies do not affect the action of this medicine.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of SOMAVERT
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the vials and on the carton after EXP. The expiry date is the last day of the month stated.
Store the vial(s) of powder in a refrigerator (between 2°C and 8°C) in its/their packaging to protect from light. Do not freeze.
The packaging(s) containing the vial(s) of SOMAVERT powder can be stored at room temperature up to a maximum of 25°C for a single period of up to 30 days. Write the expiry date on the packaging, including the day/month/year (up to 30 days from the date of removal from the refrigerator). The vial(s) must be protected from light. Do not put this medicine back in the refrigerator.
Discard this medicine if you do not use it before the new expiry date or the expiry date printed on the packaging, whichever is earlier.
Store the pre-filled syringe(s) at a temperature below 30°C or in a refrigerator (between 2°C and 8°C). Do not freeze.
After preparing the SOMAVERT solution, it must be used immediately.
Do not use this medicine if you notice that the solution is cloudy or contains particles.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
SOMAVERT Composition
- The active ingredient is pegvisomant.
- SOMAVERT 10 mg: a vial of powder contains 10 mg of pegvisomant. After reconstitution with 1 ml of solvent, 1 ml of solution contains 10 mg of pegvisomant.
- SOMAVERT 15 mg: a vial of powder contains 15 mg of pegvisomant. After reconstitution with 1 ml of solvent, 1 ml of solution contains 15 mg of pegvisomant.
- SOMAVERT 20 mg: a vial of powder contains 20 mg of pegvisomant. After reconstitution with 1 ml of solvent, 1 ml of solution contains 20 mg of pegvisomant.
- SOMAVERT 25 mg: a vial of powder contains 25 mg of pegvisomant. After reconstitution with 1 ml of solvent, 1 ml of solution contains 25 mg of pegvisomant.
- SOMAVERT 30 mg: a vial of powder contains 30 mg of pegvisomant. After reconstitution with 1 ml of solvent, 1 ml of solution contains 30 mg of pegvisomant.
- The other components are glycine, mannitol (E-421), anhydrous sodium hydrogen phosphate, and sodium dihydrogen phosphate monohydrate (see section 2 "SOMAVERT contains sodium").
- The solvent is water for injectable preparations.
Product Appearance and Container Contents
SOMAVERT is presented as a powder and solvent for injection (in a 10 mg, 15 mg, 20 mg, 25 mg, or 30 mg vial of pegvisomant and 1 ml of solvent in a pre-filled syringe). Package sizes of 1 and/or 30. Only some package sizes may be marketed.
The powder is white, and the solvent is transparent and colorless.
Marketing Authorization Holder and Manufacturer:
Marketing Authorization Holder
Pfizer Europe MA EEIG
Boulevard de la Plaine 17
1050 Bruxelles
Belgium
Manufacturer
Pfizer Manufacturing Belgium NV
Rijksweg 12
2870 Puurs-Sint-Amands
Belgium
You can request more information about this medicinal product by contacting the local representative of the marketing authorization holder:
België/Belgique/Belgien Luxembourg/Luxemburg Pfizer NV/SA Tel: +32 (0)2 554 62 11 | Lietuva Pfizer Luxembourg SARL filialas Lietuvoje Tel: +370 5 251 4000 |
Bulgaria Pfizer Bulgaria EOOD Tel: +359 2 970 4333 | Magyarország Pfizer Kft. Tel: +36 1 488 37 00 |
Ceská republika Pfizer, spol. s r.o. Tel: +420 283 004 111 | Malta Vivian Corporation Ltd. Tel: +356 21344610 |
Danmark Pfizer ApS Tlf: +45 44 20 11 00 | Nederland Pfizer bv Tel: +31 (0)800 63 34 636 |
Deutschland PFIZER PHARMA GmbH Tel: +49 (0)30 550055-51000 | Norge Pfizer AS Tlf: +47 67 52 61 00 |
Eesti Pfizer Luxembourg SARL Eesti filiaal Tel: +372 666 7500 | Österreich Pfizer Corporation Austria Ges.m.b.H. Tel: +43 (0)1 521 15-0 |
Ελλάδα Pfizer Ελλάς Α.Ε. Τηλ: +30 210 6785800 | Polska Pfizer Polska Sp. z o.o. Tel: +48 22 335 61 00 |
España Pfizer, S.L. Tel: +34 91 490 99 00 | Portugal Laboratórios Pfizer, Lda. Tel: +351 21 423 5500 |
France Pfizer Tél: +33 (0)1 58 07 34 40 | România Pfizer Romania S.R.L. Tel: +40 (0) 21 207 28 00 |
Hrvatska Pfizer Croatia d.o.o. Tel: +385 1 3908 777 | Slovenija Pfizer Luxembourg SARL Pfizer, podružnica za svetovanje s podrocja farmacevtske dejavnosti, Ljubljana Tel: +386 (0)1 52 11 400 |
Ireland Pfizer Healthcare Ireland Unlimited Company Tel: 1800 633 363 (toll free) Tel: +44 (0)1304 616161 | Slovenská republika Pfizer Luxembourg SARL, organizacná zložka Tel: +421 2 3355 5500 |
Ísland Icepharma hf. Sími: +354 540 8000 | Suomi/Finland Pfizer Oy Puh/Tel: +358 (0)9 430 040 |
Italia Pfizer S.r.l. Tel: +39 06 33 18 21 | Sverige Pfizer AB Tel: +46 (0)8 550 520 00 |
Κύπρος Pfizer Ελλάς Α.Ε. (Cyprus Branch) Τηλ: +357 22817690 | |
Latvija Pfizer Luxembourg SARL filiale Latvija Tel: +371 670 35 775 | |
Date of Last Revision of this Leaflet: 02/2025.
Other Sources of Information
Detailed information on this medicinal product is available on the European Medicines Agency website: https://www.ema.europa.eu. There are also links to other websites on rare diseases and orphan medicines.
INSTRUCTIONS FOR USE
SOMAVERT powder in vial with solvent in a pre-filled syringe
pegvisomant for injectable solution
For subcutaneous injection only
Single-dose vial
SOMAVERT is presented in a vial as a white powder block. You must mix SOMAVERT with a liquid (solvent) before you can use it.
The liquid is presented in a pre-filled syringe with the label "Solvent for SOMAVERT".
Do not mix SOMAVERT with any other liquid.
It is essential that you do not attempt to administer the injection to yourself or another person without having received training from your healthcare professional.
Store the carton containing the powder vial in the refrigerator between 2°C and 8°C and away from direct sunlight.
The packaging containing the powder vial of SOMAVERT can be stored at room temperature up to a maximum of 25°C for a single period of up to 30 days. Write the expiration date on the packaging, including the day/month/year (up to 30 days from the date of removal from the refrigerator). The vial must be protected from light. Do not put this medicine back in the refrigerator.
Discard this medicine if you do not use it before the new expiration date or the expiration date printed on the packaging, whichever comes first.
The pre-filled syringe of solvent can be stored at room temperature. Keep out of the reach of children.
- What you need
A SOMAVERT package with:
- A vial of SOMAVERT powder
- A pre-filled syringe with solvent
- A safety needle
You will also need:
- A cotton ball
- An alcohol swab
- A suitable sharps container
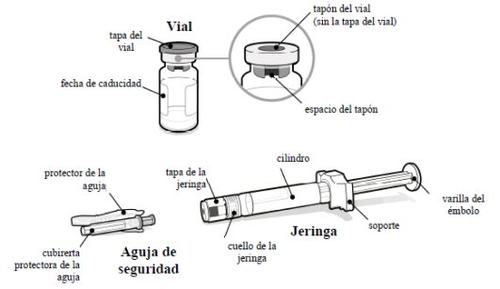
- Preparation
Before starting:
- Mix SOMAVERT with the solvent only when you are ready to inject the dose.
- Remove a single SOMAVERT package from the refrigerator and let it reach room temperature naturally in a safe place.
- Wash your hands with water and soap, and dry them well.
- Open the syringe and safety needle packaging so that each item is easier to handle while preparing for injection.
- Do not use the syringe or vial if:
- they are damaged or defective;
- the expiration date has passed;
- the syringe has been frozen, even if it has been thawed afterwards (syringe only).
- Choose an injection site
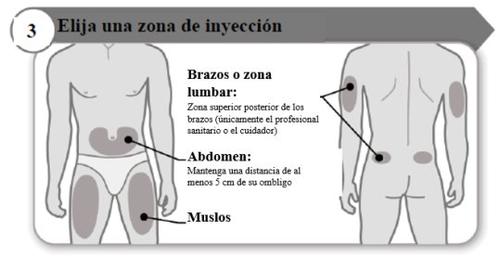
- Choose a different place within each area for the injection.
- Avoid bony areas, red, painful, or hard areas, or areas with bruises, scars, or skin diseases.
- Clean the injection site with the alcohol swab as your healthcare professional has instructed.
- Wait for the injection site to dry.
- Remove the vial cap
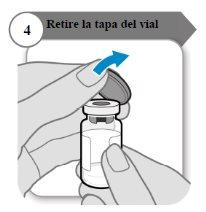
- Remove the vial cap.
- Discard the cap; it is not needed again.
Caution:Do not let anything touch the vial stopper.
- Remove the syringe cap
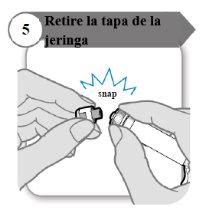
- Remove the syringe cap. You may need more force than you expect.
- Discard the syringe cap; it is not needed again.
- Keep the syringe upright to avoid spills.
Caution:Do not let the syringe tip touch anything once the cap is removed.
- Attach the safety needle
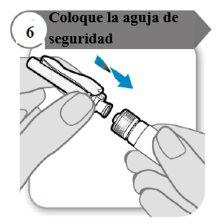
- Attach the safety needle to the syringe by twisting it firmly as much as possible.
- Remove the needle protector
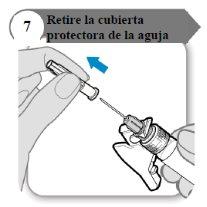
- Fold the needle protector outward, away from the needle protector.
- Carefully pull the needle protector straight off.
- Discard the needle protector; it is not needed again.
Caution:Do not let the needle touch anything.
- Insert the needle
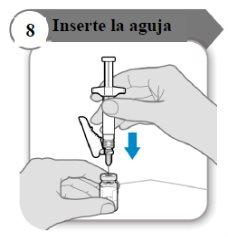
- Push the needle through the center of the vial stopper as indicated.
- Hold the syringe while the needle is inserted in the vial stopper to avoid bending the needle.
- Add the liquid
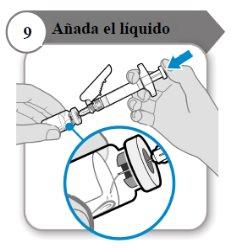
- Tilt the vial and syringe at an angle as indicated.
- Push the plunger rod slowlyuntil all the liquid is inside the vial.
- Caution:Make sure the liquid does not fall directly onto the powder, as this will form foam. The foam makes the medicine unusable.
- Do not remove the needle yet.
- Rotate the vial
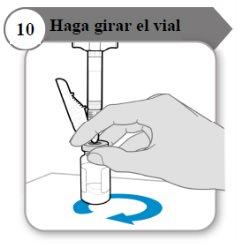
- Hold the syringe and vial with one hand as indicated.
- Gently rotate the liquid by sliding the vial in a circular motion on a flat surface.
- Continue rotating the liquid until all the powder is completely dissolved.
Note:This may take up to 5 minutes.
- Inspect the medicine
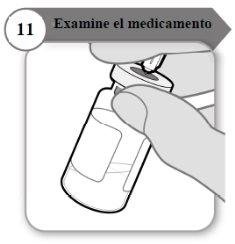
- With the needle still inserted in the vial, carefully inspect the medicine. It should be transparent and particle-free.
- Do not use it if:
- the medicine is cloudy or dark;
- the medicine has any color;
- it contains particles or there is a layer of foam in the vial.
- Reposition the needle
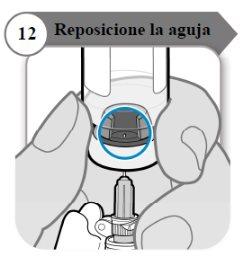
- Rotate the vial so that you can see the space in the vial stopper, as indicated.
- Pull the needle down so that the needle tip is at the lowest point in the liquid. This will help you withdraw as much liquid as possible.
- Check that the plunger rod has not moved. If it has, push it back in completely into the syringe. This ensures that all air has been removed from the syringe before withdrawing the dose.
- Withdraw the dose
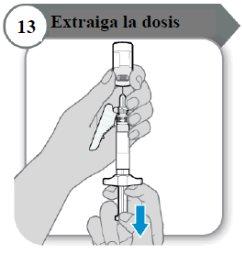
- Pull the plunger rod slowly to withdraw as much medicine as possible from the vial.
Note:If you see air in the syringe, press the syringe cylinder to make the bubbles move up, and then slowly push the bubbles back into the vial.
- Remove the needle from the vial.
- Insert the needle
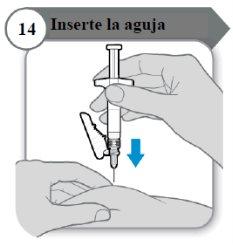
- Carefully pinch the skin at the injection site.
- Insert the needle all the way into the pinched skin.
- Inject the medicine
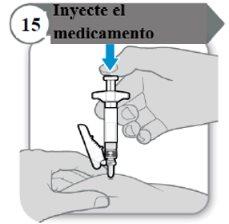
- Push the plunger rod down slowly until the syringe is empty.
Note:Make sure the needle is inserted all the way.
- Release the pinched skin and remove the needle in a straight line.
- Secure the needle
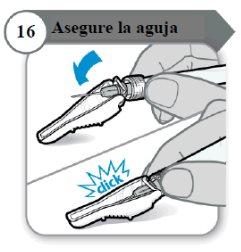
- Fold the needle protector over the needle.
- Carefullypress against a hard surface to close the needle protector.
Note:You will hear a click when the needle protector closes.
- Dispose of
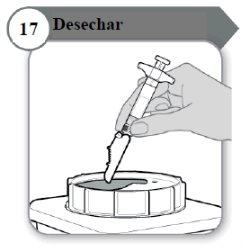
- The syringe and needle must NEVERbe reused. Dispose of the needle and syringe as your doctor, nurse, or pharmacist has instructed and according to local healthcare guidelines and safety legislation.
- After the injection
- If necessary, press lightly with a clean cotton ball on the injection site.
- Do not rub the area.
QUESTIONS AND ANSWERS
What should I do if something accidentally touches the vial stopper?
- Clean the vial stopper with a new alcohol swab and let it dry completely. If you are unable to clean the stopper, do not use the vial.
What should I do with the syringe if it has fallen?
- Do not use it, even if it appears to be undamaged. Dispose of the syringe in the same way as a used syringe. You will need another syringe.
How many times can I safely insert the needle into the vial stopper?
- Only once. Withdrawing and reinserting the needle significantly increases the risk of needle damage and may cause it to become dull. This can cause discomfort and increase the risk of skin damage and infection. There is also a risk that some of the medicine may be lost.
Is it okay to shake the vial if the powder does not dissolve?
- No, never shake the vial. Shaking can make the medicine unusable and form foam. The powder may take a few minutes to dissolve completely, so continue to move the vial slowly with circular motions until the liquid is completely transparent.
How can I tell if there is foam in the vial?
- Foam appears as a mass of small bubbles that float on top of the liquid. Do not inject SOMAVERT if foam has formed.
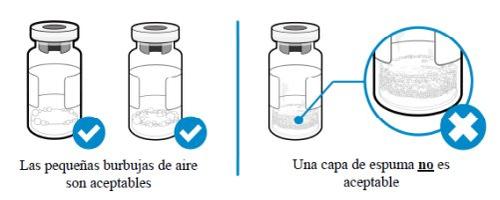
How can I avoid the medicine forming foam?
- Push the plunger very slowly so that the liquid flows smoothly into the vial. Do not let the liquid fall directly onto the powder, as this will form foam. This technique will also reduce the time needed to mix the medicine and allow you to withdraw more medicine.
Can I see some air in the syringe? Is that okay?
- Small air bubbles in the liquid are normal, and the injection is safe. However, it is possible to accidentally aspirate some air into the syringe, which must be removed before injection. Bubbles or air spaces that float on top of the liquid should be expelled back into the vial.
Why can't I withdraw all the medicine from the vial?
- The shape of the vial means that a small amount of medicine will remain in the vial. This is normal. To ensure that only a small amount of medicine remains in the vial, make sure that when you withdraw the dose, the needle tip is inserted as far as possible into the vial.
What should I do if I have any doubts about the medicine?
- All questions should be directed to a doctor, nurse, or pharmacist with experience with SOMAVERT.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to SOMAVERT 20 mg POWDER AND SOLVENT FOR INJECTABLE SOLUTIONDosage form: INJECTABLE, 10 mgActive substance: pegvisomantManufacturer: Pfizer Europe Ma EeigPrescription requiredDosage form: INJECTABLE, 15 mgActive substance: pegvisomantManufacturer: Pfizer Europe Ma EeigPrescription requiredDosage form: INJECTABLEActive substance: pegvisomantManufacturer: Pfizer Europe Ma EeigPrescription required
Online doctors for SOMAVERT 20 mg POWDER AND SOLVENT FOR INJECTABLE SOLUTION
Discuss questions about SOMAVERT 20 mg POWDER AND SOLVENT FOR INJECTABLE SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















