
SICCAFLUID 2.5 mg/g OPHTHALMIC GEL

How to use SICCAFLUID 2.5 mg/g OPHTHALMIC GEL
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
SICCAFLUID 2.5 mg/g OPHTHALMIC GEL
Carbomer 974 P
Read this package leaflet carefully because it contains important information for you.
This medicine can be obtained without a prescription. Nevertheless, to obtain the best results, it should be used properly.
- Keep this package leaflet, as you may need to read it again.
- If you need advice or more information, consult your pharmacist.
- If the symptoms worsen or persist, you should consult a doctor.
- If you consider that any of the adverse effects you are suffering from is serious or if you notice any adverse effect not mentioned in this package leaflet, inform your doctor or pharmacist.
Contents of the Package Leaflet
- What is Siccafluid and what is it used for
- Before using Siccafluid
- How to use Siccafluid
- Possible side effects
- Storage of Siccafluid
- Additional information
1. What is SICCAFLUID and what is it used for
Siccafluid is a tear substitute and contains a lubricant called Carbomer 974P.
It is an ophthalmic gel used for the relief of symptoms of dry eyes(such as inflammation, burning, irritation, or dryness) caused when the eyes do not produce enough tears.
2. Before using SICCAFLUID
Do not use Siccafluid
•If you are allergic (hypersensitive) to carbomer or any of the other components of Siccafluid listed in section 6, "What contains Siccafluid".
Be careful with Siccafluid
•If you use soft contact lenses:you should remove them before using Siccafluid. After using Siccafluid, wait at least 30 minutes before putting them back on. See also section 2, "Siccafluid contains benzalkonium chloride".
•If your condition worsens or does not improveafter starting treatment with Siccafluid, contact your doctor.
•DO NOT INJECT, DO NOT INGEST.
Children and adolescents up to 18 years of age
The safety and efficacy of SICCAFLUID in children and adolescents at the recommended posology for adults have been established based on clinical experience, but no clinical studies are available.
Use of other medications
Tell your doctor or pharmacist if you are using or have recently used other medications, including those purchased without a prescription.
If you need to use any other ophthalmic medication during treatment with Siccafluid: first use the other ophthalmic medication and wait 15 minutes before using Siccafluid.
Pregnancy and breastfeeding
Consult your doctor or pharmacist before using any medication.
If you are pregnant or breastfeeding, contact your doctor to consult before starting the use of Siccafluid.
He or she will decide if you can use Siccafluid.
Driving and using machines
Your vision may become blurry for a short time after using Siccafluid.
You should not drive or use machinery until your vision returns to normal.
Siccafluid contains benzalkonium chloride
This medication contains 0.0015 mg of benzalkonium chloride in each drop, equivalent to 0.06 mg/g.
Benzalkonium chloride can be absorbed by soft contact lenses and may alter the color of the contact lenses. Remove the contact lenses before using this medication and wait 30 minutes before putting them back on.
Benzalkonium chloride can cause eye irritation, especially if you have dry eye or other corneal diseases (transparent layer of the front of the eye). Consult your doctor if you feel a strange sensation, itching, or pain in the eye after using this medication.
.
3. How to use SICCAFLUID
If you have been recommended Siccafluid for use, follow exactly the administration instructions indicated by your doctor. Consult your doctor or pharmacist if you have any doubts.
The usual doseis 1 drop administered in the affected eye or eyes up to 4 times a day.
Children and adolescents up to 18 years of age
The safety and efficacy of SICCAFLUID in children and adolescents at the recommended posology for adults have been established based on clinical experience, but no clinical studies are available.
How to use:
Do not usethe bottle if the seal ring of the cap is brokenbefore the first opening.
Wash your hands before opening the bottle. Tilt your head back and look up.
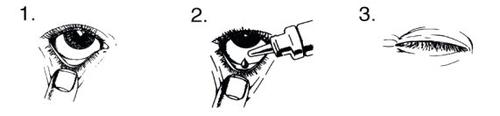
- Gently pull down the lower eyelid of the affected eye until a small "pocket" is formed.
- Turn the bottle upside down. Press until a drop falls into the "pocket".
- Release the lower eyelid and blink several times.
- Repeat steps 1 to 3 in the other eye if it also needs to be treated.
To help prevent infection, do not touch the eye, the surrounding areas, or any other area with the
dropper of the bottle.
Replace the cap on the bottle after use.
Storethe bottle in a vertical position, with the cap facing down, in order to facilitate the formation of drops for the next time you use Siccafluid.
Once opened, do not use for more than 28 days; also see section 5, "Storage of Siccafluid".
If you use more SICCAFLUID than you should
Using more drops of Siccafluid than you should does not cause any harm.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone: 91 562 04 20.
If you forget to use SICCAFLUID
Do not use a double dose to make up for the forgotten dose. Apply the next dose according to the frequency you have established.
If you have any other doubts about the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medications, Siccafluid can cause side effects, although not all people suffer from them.
Contactyour doctor if:
•The symptoms worsen or do not improve after starting treatment with Siccafluid.
If you experience any of the following side effects after applying the ophthalmic gel to the eye, talk to your doctor if it concerns you:
•Transient blurred vision
•Mild itching or burning sensation in the eye.
The mentioned side effects may occur, but the number of people likely to be affected may vary.
Reporting of side effects
If you experience any type of side effect, consult your doctor, pharmacist, or nurse, even if it is a possible side effect not listed in this package leaflet. You can also report them directly through the Spanish Medicines Monitoring System: https://notificaram.es. By reporting side effects, you can contribute to providing more information on the safety of this medication.
5. Storage of SICCAFLUID
Keep out of the reach and sight of children.
Do not use Siccafluid 2.5 mg/g ophthalmic gel after the expiration date stated on the label of the bottle and on the carton after EXP. The expiration date is the last day of the month indicated.
Do not store above 25°C. Keep the bottle in the outer packaging to protect it from light.
Storethe bottle in a vertical position, with the cap facing down, in order to facilitate the formation of drops for the next time you use Siccafluid.
Discard the bottle 28 days after the first opening, even if there are still some drops left in it.
Medicines should not be disposed of through wastewater or household waste. Deposit the packaging and unused medicines at the SIGRE collection point in the pharmacy. If in doubt, ask your pharmacist how to dispose of the packaging and unused medicines. This will help protect the environment.
6. Additional information
Composition of Siccafluid
- The active ingredient is Carbomer 974 P 2.5 mg/g.
- The other ingredients are benzalkonium chloride, sorbitol, lysine monohydrate, sodium acetate trihydrate, polyvinyl alcohol, and water for injectable preparations.
Appearance of the product and packaging content
Siccafluid is a slightly yellowish opalescent gel packaged in plastic bottles with a screw cap.
Each bottle contains 10 g of gel.
Each carton contains one bottle.
Marketing authorization holder
LABORATORIOS THEA S.A.
C/ Enric Granados, nº 86-88, 2ª planta, 08008 Barcelona
Manufacturer
URSAPHARM, ARZNEIMITTEL GMBH
Industriestrasse
66129 Saarbrücken, Germany
or
FARMILA-THEA Farmaceutici S.p.A.
Via Enrico Fermi, 50
20019 Settimo Milanese
Italy
This package leaflet was approved in March 2025
Detailed and updated information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.es/
- Country of registration
- Average pharmacy price4.06 EUR
- Availability in pharmacies
Supply issue reported
Data from the Spanish Agency of Medicines (AEMPS) indicates a supply issue affecting this medicine.<br><br>Availability may be limited in some pharmacies.<br><br>For updates or alternatives, consult your pharmacist. - Active substance
- Prescription requiredNo
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to SICCAFLUID 2.5 mg/g OPHTHALMIC GELDosage form: EYEDROP, 5.5 mg sodium chloride; 3 mg hypromellose/mlActive substance: artificial tears and other indifferent preparationsManufacturer: Alcon Healthcare S.A.Prescription not requiredDosage form: EYEDROP, 3.2 mg/mlActive substance: artificial tears and other indifferent preparationsManufacturer: Bausch & Lomb S.A.Prescription not requiredDosage form: EYE DROP, 3.2 mg/mlActive substance: artificial tears and other indifferent preparationsManufacturer: Bausch & Lomb S.A.Prescription not required
Online doctors for SICCAFLUID 2.5 mg/g OPHTHALMIC GEL
Discuss questions about SICCAFLUID 2.5 mg/g OPHTHALMIC GEL, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions









