
SAFLUTAN 15 micrograms/ml EYE DROPS IN SOLUTION IN SINGLE-DOSE CONTAINERS


How to use SAFLUTAN 15 micrograms/ml EYE DROPS IN SOLUTION IN SINGLE-DOSE CONTAINERS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
SAFLUTAN 15 micrograms/ml
eye drops, solution in single-dose container
Tafluprost
Read this entire leaflet carefully before you start using this medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the package leaflet
- What is SAFLUTAN and what is it used for
- What you need to know before you start using SAFLUTAN
- How to use SAFLUTAN
- Possible side effects
- Storage of SAFLUTAN
- Contents of the pack and further information
1. What is SAFLUTAN and what is it used for
What kind of medicine is it and how does it work?
SAFLUTAN eye drops contain tafluprost, which belongs to a group of medicines called prostaglandin analogues. SAFLUTAN reduces eye pressure. It is used when the pressure inside the eye is too high.
What is this medicine used for?
SAFLUTAN is used to treat a type of glaucoma called open-angle glaucoma, and also a condition known as ocular hypertension in adults. Both disorders are linked to an increase in the pressure inside the eye and, in the long term, can affect vision.
2. What you need to know before you start using SAFLUTAN
Do not use SAFLUTAN
- If you are allergic to tafluprost or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before you start using SAFLUTAN.
Note:SAFLUTAN may have the following effects, some of which may be permanent:
- SAFLUTAN may increase the length, thickness, color, and/or amount of eyelashes, and may cause unusual growth of the eyebrows.
- SAFLUTAN may cause darkening of the skin color around the eyes. Wipe off any excess solution from the skin. This will reduce the risk of skin darkening.
- SAFLUTAN may change the color of the iris (the colored part of the eye). If SAFLUTAN is used in only one eye, it may become a different color from the untreated eye permanently.
- SAFLUTAN may cause hair growth in areas where the solution comes into repeated contact with the skin surface.
Tell your doctor:
Children and adolescents
SAFLUTAN is not recommended for use in children and adolescents under 18 years of age, due to a lack of data on safety and efficacy.
Using SAFLUTAN with other medicines
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
If you use other medicines in the eye, wait at least 5 minutes after applying SAFLUTAN and before using the other medicine.
Pregnancy, breastfeeding, and fertility
If you can become pregnant, you should use an effective contraceptive method during treatment with SAFLUTAN. If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Driving and using machines
SAFLUTAN has no influence on the ability to drive and use machines. After applying SAFLUTAN, you may notice blurred vision for a while. Do not drive or use any tools or machines until your vision is clear.
SAFLUTAN contains phosphates
This medicine contains approximately 0.04 mg of phosphates in each drop, equivalent to 1.2 mg/ml. If you have severe corneal damage (the transparent layer of the front of the eye), treatment with phosphates, in very rare cases, may cause blurred vision due to calcium accumulation.
3. How to use SAFLUTAN
Follow the instructions for administration of this medicine exactly as indicated by your doctor or pharmacist. If you are unsure, consult your doctor or pharmacist again.
The recommended dose is one drop of SAFLUTAN in one eye or in both eyes, once a day, at night. Do not instill more drops or use it more frequently than indicated by your doctor.
This may make SAFLUTAN less effective.
Use SAFLUTAN in both eyes only if your doctor tells you to.
For use only as eye drops. Do not swallow.
Instructions for use:
When starting a new bag:
Do not use the single-dose containers if the bag is open. Open the bag along the dotted line. Write the date you opened the bag in the space provided for the date on the bag.
Each time you use SAFLUTAN:
- Wash your hands.
- Remove the strip of containers from the bag.
- Remove a single-dose container from the strip.
- Put the remaining strip back in the bag and fold the edge to close the bag.
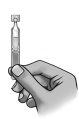 Make sure the solution is at the bottom of the single-dose container.
Make sure the solution is at the bottom of the single-dose container.
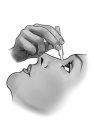
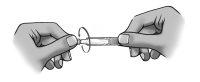 To open the container, unscrew the tab.
To open the container, unscrew the tab.
- Tilt your head back.
- Place the tip of the container close to the eye.
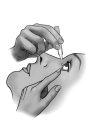 Pull the lower eyelid down and look up.
Pull the lower eyelid down and look up.
- Gently squeeze the container and let one drop fall into the space between the lower eyelid and the eye.
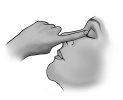 Close your eye for a moment and press the inner corner of the eye with your finger for about one minute. This helps prevent the drop from draining into the tear duct.
Close your eye for a moment and press the inner corner of the eye with your finger for about one minute. This helps prevent the drop from draining into the tear duct.- Wipe off any excess solution from the skin around the eye.
If a drop does not fall into the eye, try again.
If your doctor has told you to use eye drops in both eyes, repeat steps 7 to 12 in the other eye. The contents of one single-dose container are sufficient for both eyes. Discard the opened container with the remaining solution immediately after use.
If you use other medicines in the eye, wait at least five minutes after applying SAFLUTAN and before using the other medicine.
If you use more SAFLUTAN than you should, it is unlikely to cause serious harm. Apply the next dose at the normal time.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone: 91 562 04 20.
If you forget to use SAFLUTAN, apply one drop as soon as you remember, and return to your normal schedule. Do not use a double dose to make up for forgotten doses.
Do not stop using SAFLUTAN without consulting your doctor. If you stop treatment with SAFLUTAN, the pressure inside the eye will increase again. This can cause permanent damage to the eye.
If you have any other questions about the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. Most side effects are not serious.
Common side effects
The following effects may affect up to 1 in 10 people:
Effects on the nervous system:
- headache
Effects on the eyes:
- eye itching
- eye irritation
- eye pain
- eye redness
- changes in the length, thickness, and amount of eyelashes
- eye dryness
- feeling of having a foreign body in the eye
- change in eyelash color
- eyelid redness
- small areas of inflammation on the surface of the eye
- sensitivity to light
- watery eyes
- blurred vision
- decreased visual ability to see details
- change in iris color (may be permanent)
Uncommon side effects
The following effects may affect up to 1 in 100 people:
Effects on the eyes:
- change in skin color around the eyes
- eyelid swelling
- tired eyes
- swelling of the superficial membranes of the eye
- watery eyes
- inflammation of the eyelids
- signs of inflammation inside the eye
- eye discomfort
- pigmentation of the superficial membranes of the eye
- follicles on the superficial membranes of the eye
- allergic inflammation
- abnormal sensation in the eye
Effects on the skin and subcutaneous tissue:
- unusual hair growth on the eyelids
Frequency not known: cannot be estimated from the available data
Effects on the eyes:
- inflammation of the iris/uvea (central layer of the eye)
- sunken eyes
- macular edema/cystoid macular edema (inflammation of the retina inside the eye leading to vision impairment)
Effects on the respiratory system:
- worsening of asthma, difficult breathing
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of SAFLUTAN
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the single-dose container, the bag, and the carton after “EXP” or “CAD”. The expiry date is the last day of the month shown.
Store the unopened aluminum bags in the refrigerator (between 2°C and 8°C). Do not open the bag until you are about to use the eye drops, as the containers must be discarded 28 days after first opening the bag.
After opening the aluminum bag:
- Keep the single-dose containers in the original aluminum bag.
- Do not store above 25°C.
- Discard unused single-dose containers after 28 days from the date of first opening the aluminum bag.
- Discard the opened container with the remaining solution immediately after use.
Medicines should not be disposed of via wastewater or household waste. Return the containers and any unused medicines to the pharmacy. If you are unsure, ask your pharmacist how to dispose of the containers and any unused medicines. This will help protect the environment.
6. Contents of the pack and further information
Composition of SAFLUTAN
- The active substance is tafluprost. 1 ml of solution contains 15 micrograms of tafluprost.
- A single-dose container (0.3 ml) contains 4.5 micrograms of tafluprost. One drop (approximately 30 µl) contains approximately 0.45 micrograms of tafluprost.
- The other ingredients are glycerol, sodium dihydrogen phosphate dihydrate, disodium edetate, polysorbate 80, and water for injections. Hydrochloric acid, sodium hydroxide, or both, are added to adjust the pH.
Appearance and packaging
SAFLUTAN is a clear and colorless liquid (solution) supplied in plastic single-dose containers, each containing 0.3 ml of solution. The containers are provided in a bag of 10. SAFLUTAN is supplied in containers that contain 30 or 90 single-dose containers. Not all pack sizes may be marketed.
Marketing authorisation holder and manufacturer
Marketing authorisation holder:
Santen Oy
Niittyhaankatu 20
33720 Tampere
Finland
Manufacturer:
Santen Oy
Kelloportinkatu 1
33100 Tampere
Finland
You can obtain further information on this medicine from the local representative of the marketing authorisation holder:
Santen Pharmaceutical Spain S.L.
Acanto, 22, 7º
28045 Madrid
This medicine is authorised in the Member States of the European Economic Area and in the United Kingdom (Northern Ireland) under the following names:
Bulgaria, Czech Republic, Denmark, Estonia, Finland, Hungary, Iceland, Latvia, Lithuania, Norway, Poland, Slovakia, Sweden | Taflotan |
Germany | Taflotan sine |
Austria, Belgium, Cyprus, France, Greece, Ireland, Italy, Luxembourg, Malta, Netherlands, Portugal, Romania, Slovenia, Spain, United Kingdom (Northern Ireland) | Saflutan |
Date of last revision of this leaflet:September 2021
Detailed and up-to-date information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price28.19 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to SAFLUTAN 15 micrograms/ml EYE DROPS IN SOLUTION IN SINGLE-DOSE CONTAINERSDosage form: EYE DROP, 15 µg/mlActive substance: tafluprostManufacturer: Santen OyPrescription requiredDosage form: EYEDROP, 0.3 mg/mlActive substance: bimatoprostManufacturer: Tiedra Farmaceutica S.L.Prescription requiredDosage form: EYE DROP, 0.3 mg/mlActive substance: bimatoprostManufacturer: Sifi S.P.A.Prescription required
Online doctors for SAFLUTAN 15 micrograms/ml EYE DROPS IN SOLUTION IN SINGLE-DOSE CONTAINERS
Discuss questions about SAFLUTAN 15 micrograms/ml EYE DROPS IN SOLUTION IN SINGLE-DOSE CONTAINERS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















