
RINO-EBASTEL 10 mg/120 mg PROLONGED-RELEASE HARD CAPSULES

How to use RINO-EBASTEL 10 mg/120 mg PROLONGED-RELEASE HARD CAPSULES
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
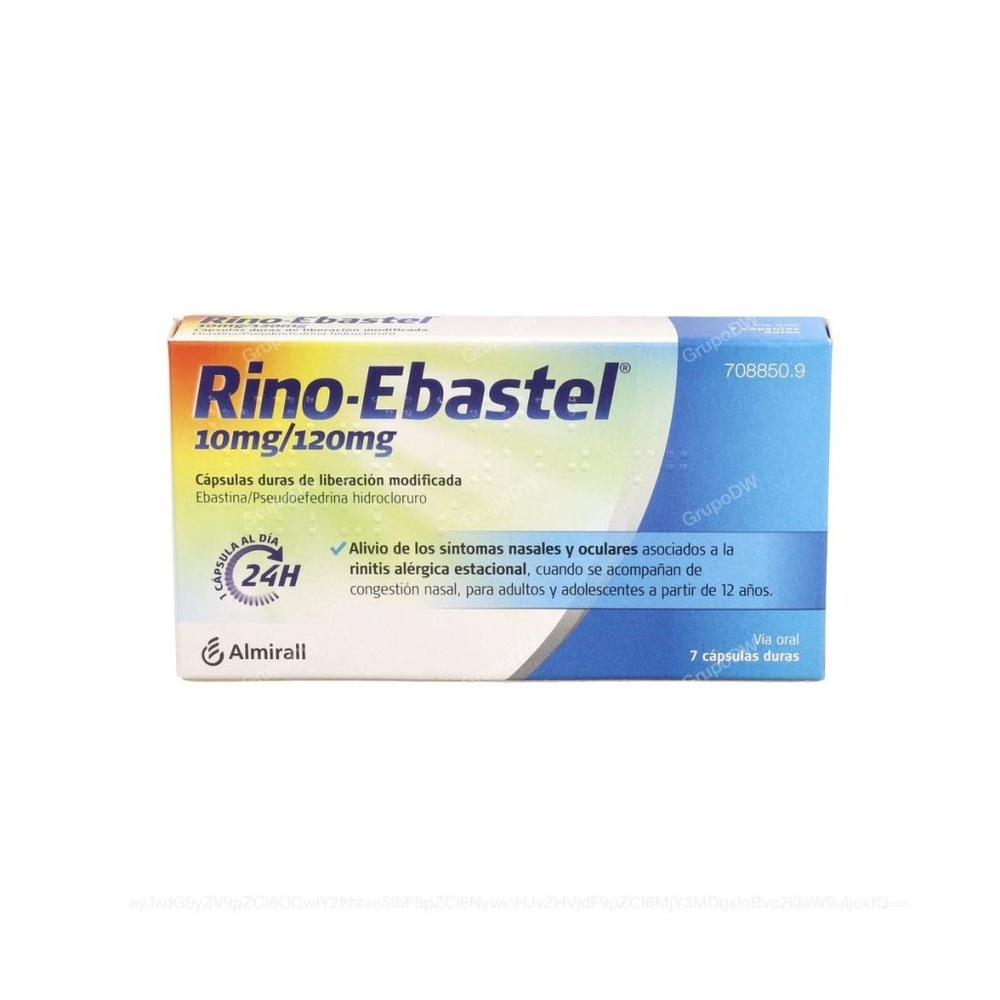
Rino-Ebastel 10 mg/120 mg Modified Release Hard Capsules
ebastine/pseudoephedrine hydrochloride
Read the entire package leaflet carefully before starting to take this medication, as it contains important information for you.
Follow the administration instructions for the medication contained in this package leaflet or as indicated by your doctor or pharmacist exactly.
- Keep this package leaflet, as you may need to read it again.
- If you need advice or more information, consult your pharmacist.
- If you experience side effects, consult your doctor or pharmacist, even if they are side effects not listed in this package leaflet. See section 4.
- You should consult a doctor if your condition worsens or does not improve after 7 days.
Contents of the Package Leaflet:
- What is Rino-Ebastel and what is it used for
- What you need to know before taking Rino-Ebastel
- How to take Rino-Ebastel
- Possible side effects
- Storage of Rino-Ebastel
- Package contents and additional information
1. What is Rino-Ebastel and what is it used for
Rino-Ebastel contains a combination of 2 active substances, ebastine and pseudoephedrine.
Ebastine is an antihistamine (anti-allergic) and pseudoephedrine is a nasal decongestant. Antihistamines help reduce allergy symptoms by preventing the effects of histamine release. Decongestants help reduce nasal congestion.
Rino-Ebastel is indicated for the relief of nasal and ocular symptoms associated with seasonal allergic rhinitis, when accompanied by nasal congestion, for adults and adolescents from 12 years of age.
You should consult a doctor if your condition worsens or does not improve after 7 days of treatment.
2. What you need to know before taking Rino-Ebastel
Do not take Rino-Ebastel
- if you have very high blood pressure (severe hypertension) or uncontrolled hypertension.
- if you have severe kidney disease, acute (sudden) or chronic (long-term), or kidney failure.
- if you are allergic to ebastine, pseudoephedrine, or any of the other components of this medication (listed in section 6).
- if you have an eye disease that causes increased pressure inside the eye and can lead to blindness (narrow-angle glaucoma).
- if you have urinary retention.
- if you have very high blood pressure (severe arterial hypertension).
- if you have decreased blood flow to the heart through the coronary arteries (coronary insufficiency).
- if you have a condition where the thyroid gland produces too much thyroid hormone (hyperthyroidism).
- if you are taking or have taken in the last 2 weeks medications used to treat depression called monoamine oxidase inhibitors (MAOIs).
- if you are in the first trimester of pregnancy.
- if you are breastfeeding
Warnings and precautions
Consult your doctor or pharmacist before starting to take Rino-Ebastel:
- if you develop a generalized febrile erythema associated with pustules, stop taking Rino-Ebastel and contact your doctor or seek medical attention immediately. See section 4.
- if you have a disease where the pressure inside the eye is increased (glaucoma).
- if you have high blood pressure (arterial hypertension).
- if you have a disease where blood sugar levels are very high (diabetes).
- if you have an enlarged prostate (prostatic hypertrophy).
- if you have any heart disease.
- if you have severe or moderate kidney disease with reduced function.
- if your electrocardiogram results are altered (prolonged QT interval).
- if you have low potassium levels in the blood.
- if you suffer from severe liver disease (see "How to take Rino-Ebastel").
- if you are being treated with a type of medication used to treat fungal infections called azole antifungals or with medications used to treat certain infections called macrolide antibiotics (see "Using Rino-Ebastel with other medications").
- if you are being treated with rifampicin, a type of medication used to treat tuberculosis.
- if you are over 60 years old.
There have been reports of posterior reversible encephalopathy syndrome (PRES) and reversible cerebral vasoconstriction syndrome (RCVS) after the use of medications containing pseudoephedrine. PRES and RCVS are rare diseases that can involve a reduction in blood flow to the brain. Stop using Rino-Ebastel immediately and seek immediate medical attention if you present symptoms that may be signs of PRES or RCVS (to know the symptoms, see section 4 "Possible side effects").
Abdominal pain or rectal bleeding may occur with the use of Rino-Ebastel due to colon inflammation (ischemic colitis). If these gastrointestinal symptoms appear, stop taking Rino-Ebastel and contact your doctor or seek medical attention immediately. See section 4.
With Rino-Ebastel, blood flow to the optic nerve may be reduced. If you suffer a sudden loss of vision, stop taking Rino-Ebastel and contact your doctor or seek medical attention immediately. See section 4.
If you take other medications that contain nasal decongestants, you should not take this medication.
You should interrupt treatment and consult your doctor if, during treatment with this medication, you notice or are diagnosed with high blood pressure (hypertension), rapid or strong heartbeats (tachycardia), palpitations, or alteration of heart rhythm (arrhythmias).
You should suspend treatment at least 24 hours before surgery.
The simultaneous use of cocaine with this medication can increase cardiovascular effects and the risk of side effects.
Use in children
Rino-Ebastel should not be administered to children under 12 years of age.
Other medications and Rino-Ebastel
Inform your doctor or pharmacist if you are taking, have recently taken, or may need to take any other medication, especially if you are taking any of the medications mentioned below, as they may interfere with Rino-Ebastel; in these cases, it may be necessary to change the dose or interrupt treatment with one of them:
- Medications used to treat depression called antidepressants type IMAO, because they can severely increase blood pressure (see "Do not take Rino-Ebastel")
- Levodopa and selegiline (medications used to treat Parkinson's disease) because they can produce a severe increase in blood pressure, very high fever, and headache.
- Nitrates (used to treat angina pectoris) because they can reduce the effects of nitrates.
- Rino-Ebastel may increase the effect of other medications used to treat allergies (antihistamines).
- Ketoconazole and itraconazole used to treat fungal infections or with an antibiotic used to treat certain infections called erythromycin (because they can produce an alteration in your electrocardiogram).
- Certain medications used to lower blood pressure (methyldopa, mecamylamine, reserpine, and veratrum alkaloids).
- The antihistamine effect of Rino-Ebastel may be decreased in patients treated with a medication called rifampicin used to treat tuberculosis.
- The administration of Rino-Ebastel together with a type of medication used to relieve nasal congestion or increase blood pressure, among others, called sympathomimetics, such as phenylephrine, methoxamine, phenylpropanolamine, ethylephrine (sympathomimetics whose action takes place from inside the body), propylhexedrine, naphazoline, oxymetazoline, tetrahydrozoline, xylometazoline, phenoxazoline, tramazoline, chlorobutanol (sympathomimetics for application on the skin or mucous membranes and have a local effect), produces an increase in the effects of these medications, which can increase their toxicity.
- Certain medications used to lower blood pressure or promote urine elimination (such as beta-blockers, ACE inhibitors, rauwolfia alkaloids like reserpine, methyldopa, mecamylamine, guanethidine, and veratrum alkaloids) because they can produce a decrease in heart rate or a sudden increase in blood pressure.
- Urinary alkalizers (sodium bicarbonate, citrates) because they can cause pseudoephedrine to be eliminated more slowly and increase its effect and toxicity.
- Inhaled anesthetics, because they can increase the risk of heart problems.
- Nervous system stimulants (amphetamines, xanthines) because they can cause nervousness, irritability, insomnia, or possibly convulsions or alteration of heart rhythm (arrhythmias).
- Digitalis glycosides (used for the heart) because they can produce alterations in heart rhythm.
- Thyroid hormones (used for thyroid diseases) because they can increase the effects of both hormones and pseudoephedrine.
Interference with diagnostic tests
Rino-Ebastel may interfere with the results of skin allergy tests, so it is recommended not to perform them until 5-7 days have passed since treatment was interrupted.
Taking Rino-Ebastel with food and drinks
The capsules can be taken with or without food. Taking this medication with food or drinks does not affect its efficacy.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medication.
Pregnancy
Rino-Ebastel is contraindicated in the first trimester of pregnancy. As a precaution, it is preferable to avoid its use throughout pregnancy.
Breastfeeding
Pseudoephedrine is excreted in breast milk, so this medication should not be used during the breastfeeding period.
Use in athletes
Athletes are informed that this medication contains a component that can produce a positive result in doping tests.
Driving and using machines
If you notice drowsiness, dizziness, or vertigo during treatment with this medication, you should not drive or use machines.
Rino-Ebastel contains sucrose, benzoic acid (E-210), and sodium
This medication contains sucrose. If your doctor has indicated that you have an intolerance to certain sugars, consult with them before taking this medication.
This medication contains 0.00016 mg of benzoic acid (E-210) (contained in the simethicone emulsion) in each capsule.
This medication contains less than 1 mmol of sodium (23 mg) per capsule; that is, it is essentially "sodium-free".
3. How to take Rino-Ebastel
Follow the administration instructions for Rino-Ebastel contained in this package leaflet or as indicated by your doctor or pharmacist exactly. In case of doubt, ask your doctor or pharmacist.
Dosage
The recommended dose in adolescents (between 12-17 years)is 1 capsule, 1 time a day, preferably with breakfast.
The recommended dose in adultsis 1 capsule, 1 time a day, preferably with breakfast. If necessary, 1 capsule can be taken every 12 hours.
In patients with severe liver disease, the dose should not exceed 1 capsule per day.
The duration of treatment should be as short as possible and should be discontinued as symptoms disappear.
You should consult your doctor if your condition worsens, if symptoms persist for more than 7 days of treatment, or if they are accompanied by high fever.
Method of administration
This medication is taken orally.
Take the capsule whole, without opening or chewing, with the help of a liquid (preferably a glass of water).
If you take more Rino-Ebastel than you should
If you take more Rino-Ebastel than you should, you may notice: rapid breathing, excitement, nervousness, irritability, restlessness, tremors, convulsions, palpitations, increased blood pressure, alteration of heart rhythm (arrhythmias), difficulty urinating. In more severe cases, it can produce: decreased potassium in the blood (hypokalemia), mental disorder with altered perception of reality (psychosis), convulsions, coma, and hypertensive crises.
In case of overdose or accidental ingestion, go immediately to a medical center or call the Toxicological Information Service (telephone: 915 620 420), indicating the medication and the amount ingested.
4. Possible side effects
Like all medications, this medication can produce side effects, although not everyone will experience them.
In clinical trials and post-marketing experience, the following side effects have been observed:
Very common: may affect more than 1 in 10 people:
- Headache
Common: may affect up to 1 in 10 people:
- Drowsiness
- Dry mouth
Rare: may affect up to 1 in 1,000 people:
- Hypersensitivity reactions: allergic reactions (such as anaphylaxis and angioedema)
- Nervousness, insomnia
- Dizziness, decreased sensation of touch or sensitivity, decreased or altered taste
- Palpitations, tachycardia
- Abdominal pain, vomiting, nausea, digestive problems
- Liver inflammation (hepatitis), cholestasis, abnormal liver function tests (increased transaminases, gamma-GT, alkaline phosphatase, and bilirubin)
- Urticaria, skin rash, dermatitis
- Menstrual disorders
- Edema (swelling due to fluid accumulation), fatigue
Very rare: may affect up to 1 in 10,000 people:
- Anxiety, agitation, tremor
- Excitability
- Hypertension
- Urinary retention
Frequency not known (cannot be estimated from available data):
- Weight gain
- Increased appetite
During the period of use of pseudoephedrine, the following side effects have been observed, whose frequency cannot be established with precision:
More frequently: nervousness, restlessness, difficulty sleeping, anxiety, tremor. Altered taste.
Less frequently: hyperactivity, hyperexcitability, dizziness, and vertigo, headache, uncoordinated movements, dilated pupils, rapid heartbeat, elevated blood pressure. Nausea, vomiting, bloody diarrhea. Dermatitis, urticaria, skin rash. Difficulty urinating. Increased sweating, paleness, and weakness.
In rare cases: hypersensitivity reactions, hallucinations, nightmares, screaming, and confusion in children. Alterations in heart rhythm and slow heartbeats. In very rare cases, infarction, and at very high doses, convulsions, confusion, and headache.
Frequency not known (cannot be estimated from available data):
- Sudden onset of fever, skin redness, or many small pustules (possible symptoms of acute generalized exanthematous pustulosis - AGEP) that may occur within the first 2 days of treatment with Rino-Ebastel. See section 2.
- Stop taking Rino-Ebastel if these symptoms appear and contact your doctor or seek medical attention immediately.
- Colon inflammation due to insufficient blood irrigation (ischemic colitis).
- Decreased blood flow to the optic nerve (ischemic optic neuropathy).
- Severe diseases that affect the blood vessels of the brain known as posterior reversible encephalopathy syndrome (PRES) and reversible cerebral vasoconstriction syndrome (RCVS).
Stop using Rino-Ebastel immediately and seek urgent medical attention if you present symptoms that may be signs of posterior reversible encephalopathy syndrome (PRES) and reversible cerebral vasoconstriction syndrome (RCVS). These include:
- sudden severe headache
- discomfort
- vomiting
- confusion
- convulsions
- changes in vision
Reporting side effects
If you experience any type of side effect, consult your doctor or pharmacist, even if it is a possible side effect not listed in this package leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medications: https://www.notificaram.es. By reporting side effects, you can contribute to providing more information on the safety of this medication.
5. Storage of Rino-Ebastel
Keep this medication out of sight and reach of children.
Do not store above 30°C.
Do not use this medication after the expiration date shown on the packaging, after CAD. The expiration date is the last day of the month indicated.
Medications should not be thrown down the drain or into the trash. Deposit the packaging and medications you no longer need at the SIGRE Point in the pharmacy. In case of doubt, ask your pharmacist how to dispose of the packaging and medications you no longer need. This way, you will help protect the environment.
6. Container Content and Additional Information
Rino-Ebastel Composition
- The active ingredients are ebastine and pseudoephedrine hydrochloride. Each capsule contains 10 mg of ebastine and 120 mg of pseudoephedrine hydrochloride.
The other components are: sucrose, corn starch, hypromellose, microcrystalline cellulose (E-460i), polyoxyethylene stearate, macrogol, glycerol polyethylene glycol oxystearate, simethicone emulsion (purified water, simethicone, polyoxyethylene sorbitan trioleate (E-436), methylcellulose (E-461), polyethylene glycol stearate, glycerol monooleate (E-471), xanthan gum (E-415), benzoic acid (E-210), sulfuric acid (E-513), and sorbic acid (E-200)), ammonio methacrylate copolymer, methacrylic acid copolymer and methyl methacrylate (1:2), dibutyl sebacate, and talc. The capsule components are: erythrosine (E-127), red iron oxide (E-172), yellow iron oxide (E-172), titanium dioxide (E-171), gelatin, and ink (shellac lacquer, propylene glycol (E-1520), sodium hydroxide (E-524), povidone (E-1201), and titanium dioxide (E-171)).
Product Appearance and Container Content
Rino-Ebastel is presented in the form of hard capsules for oral administration. The hard capsules are red in color and bear the imprint EBA PSE in white.
Each container contains 7 hard capsules.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder
Almirall, S.A.
General Mitre, 151
08022 - Barcelona
Spain
Manufacturer
Almirall Pharmaceutical Industries, S.A.
Ctra. de Martorell, 41-61
08740 Sant Andreu de la Barca – Barcelona
Spain
Date of the Last Revision of this Leaflet:April 2024
Detailed information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) (http://www.aemps.gob.es/)
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to RINO-EBASTEL 10 mg/120 mg PROLONGED-RELEASE HARD CAPSULESDosage form: MODIFIED-RELEASE TABLET, 2.5 - REVIEW mgActive substance: pseudoephedrine, combinationsManufacturer: Organon N.V.Prescription requiredDosage form: MODIFIED-RELEASE TABLET, 10mg/240mgActive substance: pseudoephedrine, combinationsManufacturer: Bayer Hispania S.L.Prescription not requiredDosage form: MODIFIED-RELEASE TABLET, 5 mg loratadine / 120 mg pseudoephedrine sulfateActive substance: pseudoephedrine, combinationsManufacturer: Bayer Hispania S.L.Prescription required
Online doctors for RINO-EBASTEL 10 mg/120 mg PROLONGED-RELEASE HARD CAPSULES
Discuss questions about RINO-EBASTEL 10 mg/120 mg PROLONGED-RELEASE HARD CAPSULES, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions











