OMLYCLO 75 mg Injectable Solution in Pre-filled Syringe


How to use OMLYCLO 75 mg Injectable Solution in Pre-filled Syringe
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Omlyclo 75 mg solution for injection in pre-filled syringe
omalizumab
This medicine is subject to additional monitoring, which will allow for quick identification of new safety information. You can help by reporting any side effects you may get. The last section of the leaflet contains information on how to report side effects.
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What Omlyclo is and what it is used for
- What you need to know before you use Omlyclo
- How to use Omlyclo
- Possible side effects
- Storing Omlyclo
- Contents of the pack and other information
1. What Omlyclo is and what it is used for
Omlyclo contains the active substance omalizumab. Omalizumab is a human protein, similar to the natural proteins produced by the body. It belongs to a class of medicines called monoclonal antibodies.
Omlyclo is used for the treatment of:
- allergic asthma
- chronic rhinosinusitis (inflammation of the nose and sinuses) with nasal polyps
Allergic asthma
This medicine is used to prevent asthma from getting worse by controlling the symptoms of severe allergic asthma in adults, adolescents and children (from 6 years of age) who are already taking asthma medicines, but whose symptoms are not adequately controlled with medicines such as high-dose inhaled steroids and inhaled beta-agonists.
Chronic rhinosinusitis with nasal polyps
This medicine is used to treat chronic rhinosinusitis with nasal polyps in adults (from 18 years of age) who are taking intranasal corticosteroids (nasal spray with corticosteroids), but whose symptoms are not well controlled with these medicines. Nasal polyps are small growths in the lining of the nose. Omlyclo helps to reduce the size of the polyps and improves symptoms including nasal congestion, loss of sense of smell, mucus in the back of the throat and nasal discharge.
Omlyclo works by blocking a substance called immunoglobulin E (IgE) that is produced by the body. IgE is involved in a type of inflammation that plays a key role in causing allergic asthma and chronic rhinosinusitis with nasal polyps.
2. What you need to know before you use Omlyclo
Do not use Omlyclo
- if you are allergic to omalizumab or any of the other ingredients of this medicine (listed in section 6).
If you think you may be allergic to any of the ingredients, tell your doctor, as you should not use Omlyclo.
Warnings and precautions
Tell your doctor before using Omlyclo:
- if you have kidney or liver problems,
- if you have a condition where your immune system attacks parts of your body (autoimmune disease),
- if you are going to travel to a region where parasitic infections are common, as Omlyclo may reduce your resistance to such infections,
- if you have had a severe allergic reaction (anaphylaxis) in the past, for example as a result of using a medicine, an insect bite or food.
Omlyclo does not treat the symptoms of acute asthma, such as a sudden asthma attack. Therefore, Omlyclo should not be used to treat this type of symptom.
Omlyclo is not intended to prevent or treat other types of allergic conditions, such as sudden allergic reactions, hyperimmunoglobulin E syndrome (inherited immune disorder), aspergillosis (lung disease caused by a fungus), food allergy, eczema or hay fever, as Omlyclo has not been studied in these conditions.
Monitor for signs of allergic reactions and other serious side effects
Omlyclo may cause serious side effects. You should monitor for signs of these effects while using Omlyclo. Seek medical attention immediately if you notice any signs that indicate a serious allergic reaction or other serious side effects. These signs are mentioned in “Serious side effects” in section 4.
Before you or someone else injects Omlyclo, or if you are not a healthcare professional, it is important that you receive training from your doctor on how to recognize the early symptoms of serious allergic reactions and how to act if they occur (see section 3, “How to use Omlyclo”). Most serious allergic reactions occur during the first three doses of Omlyclo.
Children and adolescents
Allergic asthma
Omlyclo is not recommended for children under 6 years of age. Its use in children under 6 years of age has not been studied.
Chronic rhinosinusitis with nasal polyps
Omlyclo is not recommended for children and adolescents under 18 years of age. Its use in patients under 18 years of age has not been studied.
Other medicines and Omlyclo
Tell your doctor, pharmacist or nurse if you are taking, have recently taken or might take any other medicines.
This is especially important if you are using:
- medicines to treat a parasitic infection, as Omlyclo may reduce the effect of your medicines,
- inhaled corticosteroids and other medicines for allergic asthma.
Pregnancy and breastfeeding
If you are pregnant, think you may be pregnant or are planning to have a baby, ask your doctor for advice before taking this medicine. Your doctor will discuss with you the potential benefits and risks of using this medicine during pregnancy.
Tell your doctor immediately if you become pregnant while being treated with Omlyclo.
Omlyclo may pass into breast milk. If you are breastfeeding or plan to breastfeed, ask your doctor for advice before taking this medicine.
Driving and using machines
Omlyclo is unlikely to affect your ability to drive or use machines.
3. How to use Omlyclo
Follow exactly the instructions for administration of this medicine given by your doctor. If you are unsure, ask your doctor, pharmacist or nurse again.
How to use Omlyclo
Omlyclo is used as an injection under the skin (known as a subcutaneous injection).
Omlyclo injection
- You and your doctor will decide if you will inject Omlyclo yourself. The first three doses will always be injected under the supervision of a healthcare professional (see section 2).
- It is important that you have received proper training on how to inject the medicine before you do it yourself.
- The caregiver (e.g. parents) can give the Omlyclo injection after proper training.
For detailed instructions on how to inject Omlyclo, see “Instructions for use of Omlyclo in pre-filled syringe” at the end of this leaflet.
Training to recognize serious allergic reactions
It is also important that you do not inject Omlyclo yourself until your doctor or nurse has taught you:
- how to recognize the signs and symptoms of serious allergic reactions,
- what to do if the symptoms appear.
For more information on the signs and symptoms of serious allergic reactions, see section 4.
How much to use
Your doctor will decide how much Omlyclo you need and how often you should use it. This depends on your body weight and the results of a blood test done before starting treatment to determine the level of IgE in your blood.
You will need between 1 and 4 injections at the same time. You will need injections every 2 or 4 weeks.
Continue taking your current asthma and/or nasal polyp medication while using Omlyclo. Do not stop any asthma and/or nasal polyp medication without talking to your doctor.
You may not notice an immediate improvement after starting treatment with Omlyclo. In patients with nasal polyps, the effects have been observed 4 weeks after starting treatment. In patients with asthma, it usually takes between 12 and 16 weeks for the medicine to have its full effect.
Use in children and adolescents
Allergic asthma
Omlyclo can be used in children and adolescents from 6 years of age who are already taking asthma medication, but whose asthma symptoms are not well controlled by medicines such as high-dose inhaled steroids and inhaled beta-agonists. Your doctor will tell you how much Omlyclo your child needs and how often it should be given. This will depend on the child’s weight and the results of blood tests done before starting treatment to determine the level of IgE in the blood.
Children (from 6 to 11 years of age) are not expected to inject Omlyclo themselves. However, if the doctor considers it appropriate, the caregiver can give the Omlyclo injection after proper training.
Chronic rhinosinusitis with nasal polyps
Omlyclo should not be used in children and adolescents under 18 years of age.
If you miss a dose of Omlyclo
If you have missed a visit, contact your doctor or hospital as soon as possible to reschedule.
If you have missed injecting a dose of Omlyclo, inject it as soon as you remember. Then talk to your doctor to find out when you should have your next dose.
If you stop using Omlyclo
Do not stop using Omlyclo unless your doctor tells you to. Stopping or ending treatment with Omlyclo may cause your symptoms to come back.
If you have any other questions about using this medicine, ask your doctor, pharmacist or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. The side effects caused by Omlyclo are usually mild to moderate, but can occasionally be serious.
Serious side effects:
Seek medical attention immediately if you notice any of the signs of the following side effects:
Rare (may affect up to 1 in 1,000 people)
- serious allergic reactions (including anaphylaxis). Symptoms may include rash, itching, hives, swelling of the face, lips, tongue, larynx (voice box), trachea or other parts of the body, rapid heartbeat, dizziness and slight feeling of fainting, confusion, shortness of breath, wheezing or difficulty breathing, blue-tinged skin or lips, collapse and loss of consciousness. If you have a history of serious allergic reactions (anaphylaxis) not related to Omlyclo, you may be at greater risk of developing a serious allergic reaction after using Omlyclo.
- systemic lupus erythematosus (SLE). Symptoms may include muscle pain, pain and swelling of the joints, rash, fever, weight loss and fatigue.
Frequency not known (cannot be estimated from the available data)
- Churg-Strauss syndrome or hypereosinophilic syndrome. Symptoms may include one or more of the following: swelling, pain or rash around blood vessels or lymph nodes, high level of a specific type of white blood cell (marked eosinophilia), worsening of respiratory problems, nasal congestion, heart problems, pain, numbness, tingling in the arms and legs.
- low blood platelet count with symptoms such as bleeding or bruising that occurs more easily than normal.
- serum sickness. Symptoms may include one or more of the following: joint pain with or without swelling or stiffness, rash, fever, swelling of the lymph nodes, muscle pain.
Other side effects include:
Very common (may affect more than 1 in 10 people)
- fever (in children)
Common (may affect up to 1 in 10 people)
- injection site reactions including pain, swelling, itching and redness
- upper stomach pain
- headache (very common in children)
- feeling dizzy
- joint pain (arthralgia)
Uncommon (may affect up to 1 in 100 people)
- feeling sleepy or tired
- tingling or numbness of hands or feet
- fainting, low blood pressure when sitting or standing up (postural hypotension), flushing
- sore throat, cough, acute respiratory problems
- feeling sick (nausea), diarrhea, indigestion
- itching, hives, rash, increased sensitivity of the skin to the sun
- weight gain
- flu-like symptoms
- swollen arms
Rare (may affect up to 1 in 1,000 people)
- parasitic infection
Frequency not known (cannot be estimated from the available data)
- muscle pain and joint inflammation
- hair loss
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist or nurse, even if they are not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storing Omlyclo
- Keep this medicine out of the sight and reach of children.
- Do not use this medicine after the expiry date which is stated on the label after EXP. The expiry date refers to the last day of the month shown. The carton containing the pre-filled syringe can be stored for a total of 7 days at room temperature (25 °C) before use.
- Store in the original package to protect from light.
- Store in a refrigerator (between 2 °C and 8 °C). Do not freeze.
- Do not use any packaging that is damaged or shows signs of deterioration.
6. Container Contents and Additional Information
Composition of Omlyclo
- The active ingredient is omalizumab. A 0.5 ml solution syringe contains 75 mg of omalizumab.
- The other components are L-arginine hydrochloride, L-histidine monohydrate hydrochloride, L-histidine, polysorbate 20, and water for injectable preparations.
Appearance of Omlyclo and Container Contents
Omlyclo injectable solution is presented as a clear to turbid, colorless to light brownish-yellow solution in a pre-filled syringe.
Omlyclo 75 mg injectable solution is available in containers containing 1 pre-filled syringe.
Marketing Authorization Holder
Celltrion Healthcare Hungary Kft.
1062 Budapest
Váci út 1-3. WestEnd Office Building B tower
Hungary
Manufacturer
Nuvisan France SARL
2400, Route des Colles,
06410, Biot,
France
MIDAS Pharma GmbH
Rheinstrasse 49
55218 West Ingelheim Am Rhein
Rhineland-Palatinate
Germany
Kymos S.L.
Ronda de Can Fatjó 7B
Parc Tecnològic del Vallès
08290 Cerdanyola Del Valles
Barcelona
Spain
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
Belgium/Belgique/Belgien Celltrion Healthcare Belgium BVBA Tel: + 32 1 528 7418 | Lithuania Celltrion Healthcare Hungary Kft. Tel.: +36 1 231 0493 |
| Luxembourg/Luxemburg Celltrion Healthcare Belgium BVBA Tel: + 32 1 528 7418 |
Czech Republic Celltrion Healthcare Hungary Kft. Tel: +36 1 231 0493 | Hungary Celltrion Healthcare Hungary Kft. Tel.: +36 1 231 0493 |
Denmark Celltrion Healthcare Denmark ApS Tlf: +45 3535 2989 | Malta Mint Health Ltd. Tel: +356 2093 9800 |
Germany Celltrion Healthcare Deutschland GmbH Tel: +49 303 464 941 50 | Netherlands Celltrion Healthcare Netherlands B.V. Tel: + 31 20 888 7300 |
Estonia Celltrion Healthcare Hungary Kft. Tel: +36 1 231 0493 | Norway Celltrion Healthcare Norway AS |
Spain CELLTRION FARMACEUTICA (ESPAÑA) S.L. Tel: +34 910 498 478 | Austria Astro-Pharma GmbH Tel: +43 1 97 99 860 |
Greece ΒΙΑΝΕΞ Α.Ε. Τηλ: +30 210 8009111 - 120 | Poland Celltrion Healthcare Hungary Kft. Tel.: +36 1 231 0493 |
France Celltrion Healthcare France SAS Tél.: +33 (0)1 71 25 27 00 | Portugal CELLTRION PORTUGAL, UNIPESSOAL LDA Tel: +351 21 936 8542 |
Croatia Oktal Pharma d.o.o. Tel: +385 1 6595 777 | Romania Celltrion Healthcare Hungary Kft. Tel: +36 1 231 0493 |
Ireland Celltrion Healthcare Ireland Limited Tel: +353 1 223 4026 | Slovenia OPH Oktal Pharma d.o.o. Tel.: +386 1 519 29 22 |
Iceland Celltrion Healthcare Hungary Kft. Sími: +36 1 231 0493 | Slovakia Celltrion Healthcare Hungary Kft. Tel: +36 1 231 0493 |
Italy Celltrion Healthcare Italy S.R.L. Tel: +39 0247927040 | Finland Celltrion Healthcare Finland Oy. Puh/Tel: +358 29 170 7755 |
Cyprus C.A. Papaellinas Ltd Τηλ: +357 22741741 | Sweden Celltrion Sweden AB |
Latvia Celltrion Healthcare Hungary Kft. Talr.: +36 1 231 0493 |
Date of Last Revision of this Leaflet:
Other Sources of Information
Detailed information on this medication is available on the European Medicines Agency website: http://www.ema.europa.eu
INSTRUCTIONS FOR USE OF OMLYCLO PRE-FILLED SYRINGE
Read and follow the instructions for use provided with the Omlyclo pre-filled syringe before starting to use it and each time you receive a replacement. They may contain new information.
This information does not replace consultation with your healthcare professional about your disease or treatment.
Children (from 6 to less than 12 years of age) should not self-inject Omlyclo pre-filled syringe; however, if the healthcare professional considers it appropriate, a caregiver may administer the injection after receiving proper training.
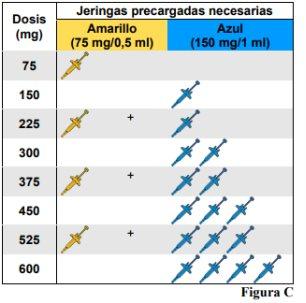
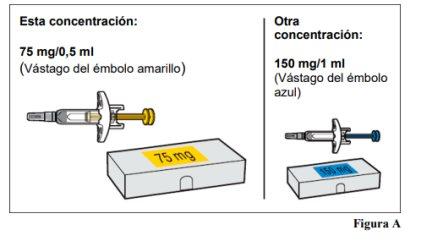
Omlyclo pre-filled syringe is available in 2 concentrations(see Figure A). These instructions should be followed for the 75 mg/0.5 ml concentration. The type of pre-filled syringe you receive will depend on the dose prescribed by your healthcare professional (see Figure C: Dosing Table). Check the label on the container and the color of the plunger rod to ensure that the concentration is correct.
Important Safety Information
- Keep the pre-filled syringe out of sight and reach of children. The pre-filled syringe contains small parts.
- Do notopen the sealed container until you are ready to use the pre-filled syringe.
- Do notuse the pre-filled syringe if the container seal or the plastic tray seal is broken, as it may not be safe to use.
- Do notleave the pre-filled syringe in a place where others may handle it.
- Do notshake the pre-filled syringe.
- Do notremove the cap until just before administering the injection.
- The pre-filled syringe cannot be reused. Dispose of the used pre-filled syringe in a puncture-resistant container immediately after use (see step 13. Disposing of the pre-filled syringe).
Storage of the Pre-filled Syringe
- Store the pre-filled syringe in the refrigerator between 2 °C and 8 °C. Keep this medication closed in its container to protect it from light.
- Do notfreeze the pre-filled syringe.
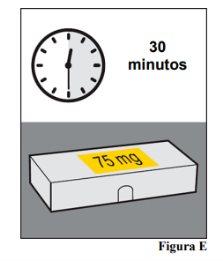
- Remember to remove the pre-filled syringe from the refrigerator and let it reach room temperature (25 °C) for about 30 minutes before preparing it for injection. Leave the pre-filled syringe in the container to protect it from light.
- The storage time of the pre-filled syringe at room temperature (25 °C) before use should not exceed 7 days.
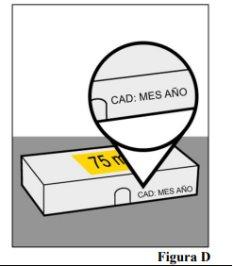
- Do notuse the pre-filled syringe after the expiration date shown on the container and on the pre-filled syringe label. If it has expired, return the entire container to the pharmacy.
- Do notuse the pre-filled syringe if it has been dropped or is visibly damaged.
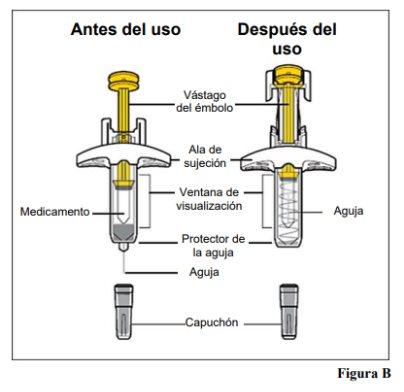
Parts of the Pre-filled Syringe (see Figure B)
Preparation for Injection
|
injection 1.a. Prepare a clean and flat surface, such as a table or counter, in a well-lit area. 1.b. Remove the container(s) containing the pre-filled syringe(s) needed to administer the prescribed dose from the refrigerator. Note: Depending on the dose prescribed by your healthcare professional, you may need to prepare one or more pre-filled syringes and inject the contents of all of them. The following table shows how many injections of each concentration are needed for the prescribed dose (see Figure C: Dosing Table). 1.c. Make sure you have the following materials:
Not included in the container:
|
|
container (see Figure D)
|
|
3.a. Leave the unopened container containing the pre-filled syringe at room temperature (25 °C) for 30 minutes to warm up (see Figure E).
|
|
4.a. Wash your hands with soap and water and dry them well (see Figure F). |
|
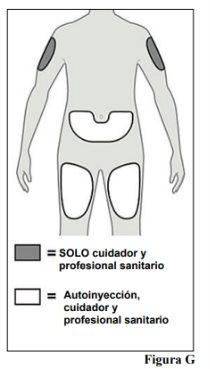
5.a. You can inject into:
5.b. Choose a different injection site for each new injection, at a minimum distance of 2.5 cm from the area used for the last injection. |
|
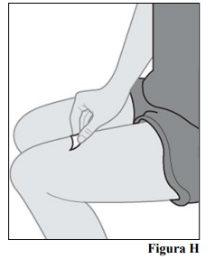
6.a. Clean the injection site with an alcohol swab using a circular motion (see Figure H). 6.b. Let the skin dry before injecting.
|
|
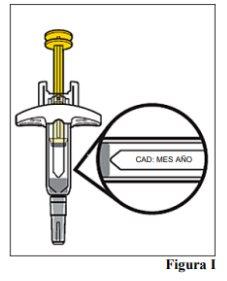
7.a. Open the container. Hold the body of the pre-filled syringe to lift it out of the tray. 7.b. Examine the pre-filled syringe and ensure it has the correct medication (Omlyclo) and dose. 7.c. Look at the pre-filled syringe and make sure it is not cracked or damaged. 7.d. Check the expiration date on the pre-filled syringe label (see Figure I).
Note: If the expiration date is not visible in the viewing window, you can turn the inner cylinder of the pre-filled syringe until the expiration date is visible. |
|
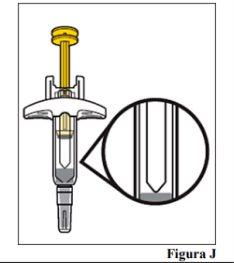
8.a. Look at the medication and confirm that the liquid is clear to turbid, colorless to light brownish-yellow, and has no particles (see Figure J).
|
Administering the Injection
|
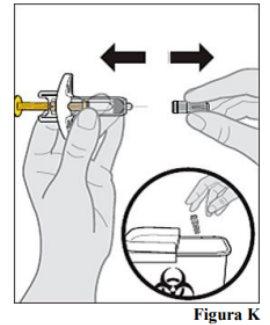
9.a. Hold the pre-filled syringe by the body with one hand. With the other hand, gently pull the cap to remove it.
9.b. Dispose of the cap immediately in a puncture-resistant container (see step 13. Disposing of the pre-filled syringeand Figure K).
|
|
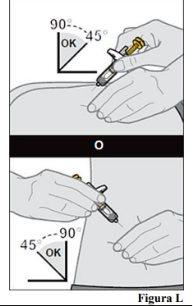
injection site. 10.a. Gently pinch a skin fold at the injection site with one hand. Note:It is important to pinch the skin to ensure that you inject under the skin (in the fatty area) but not too deeply (in the muscle). 10.b. With a quick and dart-like motion, insert the needle completely into the skin fold at an angle of 45 to 90 degrees (see Figure L).
|
|
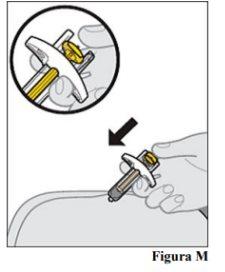
11.a. Once the needle is inserted, release the skin fold. 11.b. Slowly push the plunger rod downwarduntil the entire dose of the medication is injected and the syringe is empty (see Figure M).
|
|
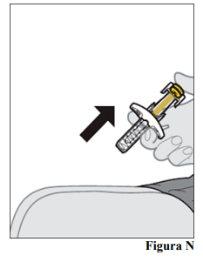
injection site 12.a. Once the pre-filled syringe is empty, slowly lift your thumb off the plunger rod until the needle is completely covered by the needle shield (see Figure N).
|
After the Injection
|
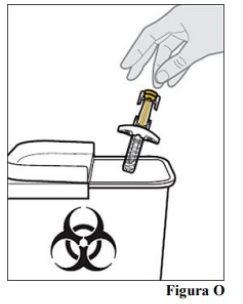
13.a. Place the used pre-filled syringe in a puncture-resistant container immediately after use (see Figure O).
in household trash. If you do not have a sharps container, you may use a household container that can be closed and is puncture-resistant. For your safety and health and that of others, used needles and syringes should never be reused. The disposal of unused medication and all materials that have come into contact with it will be carried out in accordance with local regulations.
Ask your pharmacist how to dispose of the packaging and medicines you no longer need. This will help protect the environment. |
14.a. If bleeding occurs, treat the injection site by applying gentle pressure, without rubbing, with a cotton ball or gauze at the site and apply an adhesive dressing if necessary. |
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to OMLYCLO 75 mg Injectable Solution in Pre-filled SyringeDosage form: INJECTABLE, 150 mgActive substance: omalizumabManufacturer: Celltrion Healthcare Hungary Kft.Prescription requiredDosage form: INJECTABLE, 150 mgActive substance: omalizumabManufacturer: Novartis Europharm LimitedPrescription requiredDosage form: INJECTABLE, 150 mgActive substance: omalizumabManufacturer: Novartis Europharm LimitedPrescription required
Online doctors for OMLYCLO 75 mg Injectable Solution in Pre-filled Syringe
Discuss questions about OMLYCLO 75 mg Injectable Solution in Pre-filled Syringe, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions