
METHERGIN 0.2 mg/ml INJECTABLE SOLUTION

How to use METHERGIN 0.2 mg/ml INJECTABLE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
- Introduction
- What Methergín 0.2 mg/ml Solution for Injection is and what it is used for
- What you need to know before you use Methergín 0.2 mg/ml Solution for Injection
- How to use Methergín 0.2 mg/ml Solution for Injection
- Possible side effects
- Storage of Methergín 0.2 mg/ml Solution for Injection
- Contents of the pack and other information
Introduction
Package Leaflet: Information for the Patient
METHERGÍN 0.2 mg/ml Solution for Injection
metilergometrine maleate
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the Package Leaflet
- What Methergín 0.2 mg/ml Solution for Injection is and what it is used for
- What you need to know before you use Methergín 0.2 mg/ml Solution for Injection
- How to use Methergín 0.2 mg/ml Solution for Injection
- Possible side effects
- Storage of Methergín 0.2 mg/ml Solution for Injection
- Contents of the pack and other information
1. What Methergín 0.2 mg/ml Solution for Injection is and what it is used for
Methergín contains metilergometrine as the active substance. It belongs to a group of medicines that work by stimulating the uterus, increasing the frequency and duration of uterine contractions.
Methergín is used:
- when there is loss of muscle tone of the uterus and hemorrhages after childbirth
- in scheduled deliveries, for the expulsion of the placenta
- uterine hemorrhages during cesarean sections
- hemorrhages after childbirth, when the uterus does not decrease in size or clots appear in the uterus after childbirth
- hemorrhages from abortion, initiated and incomplete abortions, and curettage
Methergín should not be used to induce or accelerate labor.
2. What you need to know before you use Methergín 0.2 mg/ml Solution for Injection
Do not use Methergín
- if you are allergic to metilergometrine, to ergot alkaloids, or to any of the other ingredients of this medicine (listed in section 6)
- if you are pregnant or think you may be pregnant
- if you are in the dilation phase of labor or before the baby's shoulder appears
- if you have very high blood pressure
- if you have a severe complication of pregnancy associated with high blood pressure, edema, protein in the urine, and convulsions (pre-eclampsia or eclampsia)
- if you have diseases caused by lack of blood flow (including angina pectoris)
- if you have a blood infection
Warnings and precautions
Consult your doctor or pharmacist before starting to use Methergín.
If any of these circumstances apply to you, tell your doctor:
- if you have high blood pressure
- if you have a heart disease (particularly one that affects the arteries that connect to the heart) or if you are at risk of suffering from a heart disease (due to obesity, diabetes, high cholesterol, or smoking)
- if you have any liver or kidney disease
Children
Methergín is not for use in children. Accidental administrations in newborns have been reported to have serious consequences.
Using Methergín with other medicines
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
It is important that you inform your doctor if you are using:
- macrolide antibiotics such as erythromycin, clarithromycin, and troleandomycin
- medicines used to treat HIV infection (antiretrovirals), such as ritonavir, nelfinavir, indinavir, delavirdine, or nevirapine
- medicines used to treat fungal infections, such as itraconazole or voriconazole
- vasoconstrictor medicines, including triptans (used for migraines) or those containing ergot alkaloids, such as ergotamine, or beta-blockers (medicines used for heart rhythm disorders and after a heart attack)
- bromocriptine (a medicine used to inhibit lactation)
- anesthetics (including local anesthetics)
- prostaglandins (used to contract the uterus)
- glyceryl trinitrate or other medicines used for angina pectoris
- rifampicin (an antibiotic)
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Ask your doctor or pharmacist for advice before using any medicine.
Methergín should not be administered to pregnant women due to its potent uterine stimulant action.
Methergín passes into breast milk. Given the possible effects on the baby and the reduction of milk secretion caused by this medicine, breastfeeding is not recommended while using Methergín. Breast milk should be discarded during treatment with Methergín until 12 hours have passed since the last administration.
Driving and using machines
Methergín may cause dizziness and convulsions, so caution should be exercised when driving, using machinery, or performing any activity that requires concentration.
Methergín contains sodium
This medicine contains less than 23 mg of sodium (1 mmol) per 5 ml (maximum daily dose); i.e., it is essentially "sodium-free".
3. How to use Methergín 0.2 mg/ml Solution for Injection
Follow exactly the administration instructions of this medicine given by your doctor. If in doubt, consult your doctor or pharmacist again.
Both for scheduled deliveries and for loss of uterine muscle tone, the recommended route of administration of Methergín is intramuscular.
Scheduled deliveries
The recommended route of administration of Methergín is intramuscular.
1 ml (0.2 mg) injected into the muscle or 0.5 to 1 ml (0.1 to 0.2 mg) by slow intravenous injection when the baby's shoulder appears, or at most immediately after the baby is born.
In some cases, it may be convenient to manually assist in the expulsion of the placenta.
In the case of delivery with anesthesia, the recommended dose is 1 ml (0.2 mg) injected slowly into the vein.
Loss of uterine muscle tone
The recommended route of administration of Methergín is intramuscular.
1 ml (0.2 mg) intramuscularly or 0.5 to 1 ml (0.1 to 0.2 mg) by slow intravenous injection. It can be repeated every 2-4 hours if necessary, up to 5 times in 24 hours.
Cesarean section
After the baby is delivered, 1 ml intramuscularly or 0.5 to 1 ml intravenously or into the uterine muscle.
Hemorrhages, when the uterus does not decrease in size or clots appear in the uterus
0.5 to 1 ml (0.1 to 0.2 mg) intramuscularly or subcutaneously up to 3 times a day for 5 days.
At the end of the package leaflet, in the section "Instructions for the correct administration of Methergín", detailed instructions for use are provided.
If you use more Methergín than you should
If you think you have been given too much Methergín, inform your doctor, nurse, or pharmacist immediately or go to the nearest hospital.
Too high a dose can cause nausea, vomiting, changes in blood pressure, numbness, tingling, and pain in the limbs, difficulty breathing, convulsions, and loss of consciousness.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service. Phone 91 562 04 20, indicating the medicine and the amount ingested.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
These side effects occur more frequently when the intravenous route is used and mainly affect the uterine muscles, digestive system, blood vessels, and bronchi.
- Common (may affect up to 1 in 10 people):
- headache
- high blood pressure
- skin rash
- abdominal pain (caused by uterine contractions)
- Uncommon (may affect up to 1 in 100 people):
- convulsions (seizures). If this symptom occurs, consult your doctor immediately or go to the emergency department of the nearest hospital
- chest pain. If this symptom occurs, consult your doctor immediately or go to the emergency department of the nearest hospital
- nausea, vomiting
- dizziness
- low blood pressure
- increased sweating
- Rare (may affect up to 1 in 1,000 people):
- slow heart rate
- fast heart rate
- palpitations
- numbness or tingling in fingers or toes and cold and pale hands or feet. If these symptoms occur, consult your doctor immediately or go to the emergency department of the nearest hospital
- Very rare (may affect up to 1 in 10,000 people):
- ringing in the ears
- nasal congestion
- diarrhea
- muscle cramps
- breathing difficulties of unknown origin, severe chest pain, mental confusion. If these symptoms occur, consult your doctor immediately or go to the emergency department of the nearest hospital
- signs of allergy, such as a sudden drop in blood pressure, flushing, and/or generalized swelling. If these symptoms occur, consult your doctor immediately or go to the emergency department of the nearest hospital
- hallucinations. If this symptom occurs, consult your doctor immediately or go to the emergency department of the nearest hospital
- swelling, redness, or pain at the injection site due to a blockage of a vein (symptoms of thrombophlebitis). If these symptoms occur, consult your doctor immediately or go to the emergency department of the nearest hospital
- Frequency not known (cannot be estimated from the available data):
- weakness or paralysis of the limbs, lips, or face, difficulty speaking (signs of stroke). If these symptoms occur, consult your doctor immediately or go to the emergency department of the nearest hospital
- angina pectoris. If this symptom occurs, consult your doctor immediately or go to the emergency department of the nearest hospital
- very fast and/or irregular heartbeat. If these symptoms occur, consult your doctor immediately or go to the emergency department of the nearest hospital
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Medicines and Healthcare Products Agency (AEMPS) website (www.notificaRAM.es). By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Methergín 0.2 mg/ml Solution for Injection
Keep this medicine out of the sight and reach of children.
Store in the outer packaging to protect from light. Store in a refrigerator (between 2 and 8°C). Do not freeze. The ampoules can be stored outside the refrigerator for 14 days at a temperature not exceeding 25°C.
Do not use this medicine after the expiry date stated on the carton after EXP. The expiry date is the last day of the month indicated.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Contents of the pack and other information
Composition of Methergín 0.2 mg/ml Solution for Injection
- The active substance is metilergometrine maleate 0.20 mg (in 1 ml of solution for injection).
- The other ingredients are: maleic acid, sodium chloride, and water for injections.
Appearance and packaging of the product
Methergín Solution for Injection is presented in 1 ml ampoules containing a clear and colorless solution for injection.
Marketing authorization holder
Essential Pharma Limited,
Vision Exchange Building
Triq it-Territorjals, Zone 1,
Central Business District,
Birkirkara, CBD 1070,
Malta
Manufacturer
Novartis Farmacéutica, S.A.
Ronda Santa María 158
Barberà del Vallès
08210 – Barcelona
Date of last revision of this leaflet:December 2020
Other sources of information
Detailed information on this medicine is available on the website of the Spanish Agency for Medicines and Healthcare Products (AEMPS) http://www.aemps.gob.es/.
This information is intended only for healthcare professionals:
See also section 3 "How to use Methergín 0.2 mg/ml Solution for Injection".
Instructions for the correct administration of Methergín
Intramuscular injections are more recommended than intravenous injections. In the case of intravenous injection, Methergín Solution for Injection should be administered slowly over a period of not less than 60 seconds. Intra- or periarterial injection should be avoided.
Administer only if the solution is clear and colorless.
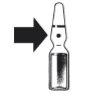
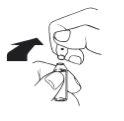
Breakable ampoule. Place your thumb above the colored break point and break the ampoule by pressing downwards.
- Country of registration
- Average pharmacy price1.33 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to METHERGIN 0.2 mg/ml INJECTABLE SOLUTIONDosage form: TABLET, 0.125 mgActive substance: methylergometrineManufacturer: Essential Pharma LimitedPrescription requiredDosage form: TABLET, 25 microgramsActive substance: misoprostolManufacturer: Norgine B.V.Prescription requiredDosage form: VAGINAL SUPPOSITORY/CAPSULE/TABLET, 200 µgActive substance: misoprostolManufacturer: Laboratorios Bial S.A.Prescription required
Online doctors for METHERGIN 0.2 mg/ml INJECTABLE SOLUTION
Discuss questions about METHERGIN 0.2 mg/ml INJECTABLE SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions






