
LUMIGAN 0.3 mg/ml EYE DROPS IN SOLUTION SINGLE-DOSE CONTAINERS

How to use LUMIGAN 0.3 mg/ml EYE DROPS IN SOLUTION SINGLE-DOSE CONTAINERS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
LUMIGAN 0.3mg/ml, eye drops solution in single-dose container
bimatoprost
Read this package leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this package leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the package leaflet:
- What LUMIGAN 0.3 mg/ml single-dose is and what it is used for
- What you need to know before you use LUMIGAN 0.3 mg/ml single-dose
- How to use LUMIGAN 0.3 mg/ml single-dose
- Possible side effects
- Storage of LUMIGAN 0.3 mg/ml single-dose
- Contents of the pack and other information
1. What LUMIGAN 0.3 mg/ml single-dose is and what it is used for
LUMIGAN 0.3 mg/ml single-dose is a medicine for glaucoma. LUMIGAN belongs to a group of medicines called prostamides.
LUMIGAN 0.3 mg/ml eye drops single-dose is used to reduce high pressure in the eye. This medicine can be used alone or with other eye drops called beta-blockers that also reduce pressure.
The eye contains a clear, watery liquid that keeps the inside of the eye healthy. This liquid is constantly drained out of the eye and new liquid is produced to replace it. If the liquid does not drain out of the eye quickly enough, the pressure inside the eye increases. This medicine works by increasing the drainage of the liquid. This reduces the pressure inside the eye. If this pressure is not reduced, it could lead to a disease called glaucoma and damage your sight.
This medicine does not contain preservatives.
2. What you need to know before you start using LUMIGAN 0.3 mg/ml single-dose
Do not use this medicine:
- If you are allergic to bimatoprost or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions:
Consult your doctor or pharmacist before starting treatment with LUMIGAN 0.3 mg/ml single-dose.
Tell your doctor or pharmacist if:
- you have any respiratory problems.
- you have liver or kidney problems.
- you have had cataract surgery in the past.
- you have low blood pressure or a slow heart rate.
- you have had a viral infection or inflammation of the eye.
During treatment, LUMIGAN may cause loss of fat around the eye, which can cause deepening of the eyelid sulcus, sinking of the eyes (enophthalmos), drooping of the eyelids (ptosis), stretching of the skin around the eye (involution of the dermatocalasis), and the white part of the eye becoming more visible (inferior scleral show). The changes are usually mild, but if they become more pronounced, they can affect your field of vision. The changes may disappear if you stop using LUMIGAN. LUMIGAN 0.3 mg/ml single-dose may also cause darkening and growth of the eyelashes, as well as darkening of the skin around the eyelid. The color of the iris may darken. These changes may be permanent and more noticeable if only one eye is being treated.
Children and adolescents
LUMIGAN 0.3 mg/ml single-dose has not been studied in patients under 18 years of age and should not be used in patients under 18 years of age.
Other medicines and LUMIGAN 0.3 mg/ml single-dose
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
LUMIGAN 0.3 mg/ml single-dose may pass into breast milk, so you should not use it if you are breastfeeding.
Driving and using machines
After instilling LUMIGAN 0.3 mg/ml single-dose, blurred vision may occur for a short time. Do not drive or use machines until you can see clearly.
3. How to use LUMIGAN 0.3 mg/ml single-dose
Follow the instructions for administration of this medicine exactly as told by your doctor or pharmacist. If you are unsure, ask your doctor or pharmacist.
The recommended dose is one drop in each eye that needs treatment, once a day, in the evening. LUMIGAN 0.3 mg/ml single-dose should only be used in the eye.
If you are using LUMIGAN 0.3 mg/ml single-dose with another eye medicine, wait at least 5 minutes between using LUMIGAN 0.3 mg/ml single-dose and the other eye medicine.
Do not use the medicine more than once a day, as this may reduce the effectiveness of the treatment.
Wash your hands before using it. Make sure the single-dose container is intact before using this medicine. The solution should be used immediately after opening the container. To avoid contamination, do not let the tip of the single-dose container touch the eye or any other surface.
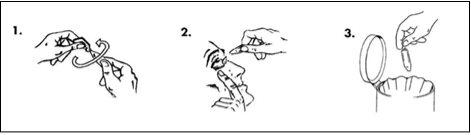
- Take a single-dose container from the bag and hold it upright (with the cap facing up) and twist the cap until it comes off.
- Pull the lower eyelid down to form a pocket. Turn the single-dose container upside down and squeeze until one drop falls into the eye or eyes to be treated.
- Discard the single-dose container after use, even if there is still solution left in it.
Wipe away any excess that runs onto your cheek.
If you wear contact lenses, you should remove them before using this medicine. Wait 15 minutes after using the drops before putting your lenses back in.
If you use more LUMIGAN 0.3 mg/ml single-dose than you should
If you use more of this medicine than you should, it is unlikely to cause you any serious harm. Apply the next dose at the usual time. If you are concerned, talk to your doctor or pharmacist.
If you forget to use LUMIGAN 0.3 mg/ml single-dose
If you forget to use this medicine, use one drop as soon as you remember and then go back to your usual routine. Do not use a double dose to make up for the forgotten dose.
If you stop using LUMIGAN 0.3 mg/ml single-dose
LUMIGAN 0.3 mg/ml single-dose should be used every day to work properly. If you stop using LUMIGAN 0.3 mg/ml single-dose, the pressure in your eye may increase, so talk to your doctor before stopping treatment.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Very common side effects
These may affect up to 1 in 10 people
Affecting the eye
- Mild redness (up to 24% of people) Loss of fat in the eye area, which can cause deepening of the eyelid sulcus, sinking of the eyes (enophthalmos), drooping of the eyelids (ptosis), stretching of the skin around the eye (involution of the dermatocalasis), and the white part of the eye becoming more visible (inferior scleral show).
Common side effects
These may affect up to 1 in 100 people
Affecting the eye
- Small erosions on the surface of the eye, with or without inflammation.
- Irritation.
- Itching in the eyes.
- Pain.
- Dryness.
- Feeling of having something in the eye.
- Longer eyelashes.
- Darker skin around the eye.
- Red eyelids.
Uncommon side effects
These may affect up to 1 in 1,000 people
Affecting the eye
- Tired eyes.
- Sensitivity to light.
- Darker iris.
- Inflamed and itchy eyelids.
- Excessive tearing.
- Inflammation of the clear layer on the front of the eye.
- Blurred vision.
Affecting the body
- Headaches.
- Growth of hair around the eye.
Side effects with unknown frequency
Affecting the eye
- Sticky eyes.
- Eye discomfort.
Affecting the body
- Asthma.
- Worsening of asthma.
- Worsening of chronic obstructive pulmonary disease (COPD).
- Difficulty breathing.
- Symptoms of an allergic reaction (inflammation, redness of the eye, and skin rash).
- Dizziness.
- High blood pressure.
- Discoloration of the skin (periocular).
In addition to the side effects of LUMIGAN 0.3 mg/ml single-dose, the following side effects have been seen with the multidose formulation of LUMIGAN 0.3 mg/ml with preservative and may occur in patients using LUMIGAN 0.3 mg/ml single-dose:
- Burning sensation in the eye.
- Allergic reaction in the eye.
- Inflammation of the eyelid.
- Difficulty seeing clearly.
- Worsening of vision.
- Darker eyelashes.
- Retinal hemorrhage.
- Inflammation inside the eye.
- Cystoid macular edema (inflammation of the retina inside the eye that leads to worsening of vision).
- Inflammation of the iris.
- Twitching of the eyelid.
- The eyelid has contracted and separated from the surface of the eye.
- Nausea.
- Redness of the skin around the eye.
- Weakness.
- Increased levels of liver enzymes in the blood.
Other side effects reported with phosphate-containing eye drops:
In very rare cases, some patients with severe damage to the clear layer on the front of the eye (the cornea) have developed cloudy patches on the cornea due to calcium deposits during treatment.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of LUMIGAN 0.3 mg/ml single-dose
Keep this medicine out of the sight and reach of children.
This medicine is for single use and does not contain preservatives. Do not store the unused solution.
Do not use this medicine after the expiry date which is stated on the single-dose container and on the carton after EXP. The expiry date is the last day of the month shown.
This medicine does not require any special storage conditions. However, once the bag is opened, it should be used within 30 days.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Contents of the pack and other information
Composition of LUMIGAN 0.3 mg/ml single-dose
- The active substance is bimatoprost. One ml of solution contains 0.3 mg of bimatoprost.
- The other ingredients are sodium chloride, disodium phosphate heptahydrate, citric acid monohydrate, and purified water. Small amounts of hydrochloric acid or sodium hydroxide may be added to maintain the normal level of acidity (pH levels).
Appearance and packaging of the product
LUMIGAN 0.3 mg/ml single-dose is a clear and transparent solution supplied in plastic single-dose containers, each containing 0.4 ml of solution.
The carton contains 5 single-dose containers in a carton.
The carton contains 3 or 9 aluminum bags, each with 10 single-dose containers, with a total of 30 or 90 single-dose containers in the carton, respectively.
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer:
AbbVie Deutschland GmbH & Co. KG
Knollstraße
67061 Ludwigshafen
Germany
For further information about this medicine, please contact the local representative of the marketing authorization holder.
Belgium/Belgique/Belgien Luxembourg/Luxemburg/Netherlands Allergan n.v. Tel: +32 (0)2 351 24 24 | Iceland Actavis Pharmaceuticals Iceland ehf. Phone: +354 550 3300 |
Bulgaria Allergan Bulgaria EOOD Phone: +359 (0) 800 20 280 | Italy Allergan S.p.A. Phone: +39 06 509 562 90 |
Czech Republic Allergan CZ s.r.o. Phone: +420 800 188 818 | Latvia Allergan Baltics UAB Phone: +371 676 60 831 |
Denmark/Norway/Finland/Sweden Allergan Norden AB Phone: +45 80 88 45 60 (DK) +47 80 01 04 97 (NO) +358 800 115 003 (FI) +46 (0)8 594 100 00 (SE) | Lithuania Allergan Baltics UAB Phone: +37 0 52 072 777 |
Germany Pharm-Allergan GmbH Phone: +49 69 92038 1050 | Hungary Allergan Hungary Kft. Phone: +36 80 100 101 |
Estonia Allergan Baltics UAB Phone: +37 2 634 6109 | Austria Pharm-Allergan GmbH Phone: +43 1 99460 6355 |
Greece/Cyprus Allergan Hellas Pharmaceuticals S.A. Phone: +30 210 74 73 300 | Poland Allergan Sp. z o.o. Phone: +48 22 256 37 00 |
Spain Allergan S.A. Phone: +34 91 807 6130 | Portugal Profarin Lda. Phone: +351 21 425 3242 |
France Allergan France SAS Phone: +33 (0)1 49 07 83 00 | Romania Allergan S.R.L. Phone: +40 21 301 53 02 |
Croatia Ewopharma d.o.o. Phone: +385 1 6646 563 | Slovenia Ewopharma d.o.o. Phone: +386 (0) 590 848 40 |
Ireland/Malta Allergan Pharmaceuticals Ireland Phone: +353 1800 931 787 (IE) +356 27780331 (MT) | Slovakia Allergan SK s.r.o. Phone: +421 800 221 223 United Kingdom Allergan Ltd. Phone: +44 (0) 1628 494026 |
Date of last revision of this package leaflet:<{MM/AAAA}>
Detailed information on this medicine is available on the European Medicines Agency website: http://www.ema.europa.eu.
The package leaflet for this medicine can be found on the European Medicines Agency website in all EU/EEA languages.
- Country of registration
- Average pharmacy price20.87 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to LUMIGAN 0.3 mg/ml EYE DROPS IN SOLUTION SINGLE-DOSE CONTAINERSDosage form: EYEDROP, 0.3 mg/mlActive substance: bimatoprostManufacturer: Tiedra Farmaceutica S.L.Prescription requiredDosage form: EYE DROP, 0.3 mg/mlActive substance: bimatoprostManufacturer: Sifi S.P.A.Prescription requiredDosage form: EYEDROP, 0.3 mg/mlActive substance: bimatoprostManufacturer: Laboratorio Stada S.L.Prescription required
Online doctors for LUMIGAN 0.3 mg/ml EYE DROPS IN SOLUTION SINGLE-DOSE CONTAINERS
Discuss questions about LUMIGAN 0.3 mg/ml EYE DROPS IN SOLUTION SINGLE-DOSE CONTAINERS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















