
GANFORT 0.3 mg/mL + 5 mg/mL Eye Drops, Solution

How to use GANFORT 0.3 mg/mL + 5 mg/mL Eye Drops, Solution
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
GANFORT 0.3mg/ml + 5mg/ml eye drops, solution
Bimatoprost/timolol
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack and other information:
- What is GANFORT and what is it used for
- What you need to know before you use GANFORT
- How to use GANFORT
- Possible side effects
5 Storage of GANFORT
- Contents of the pack and further information
1. What is GANFORT and what is it used for
GANFORT contains two different active substances (bimatoprost and timolol) that reduce high pressure in the eye. Bimatoprost belongs to a group of medicines called prostamides, a prostaglandin analogue. Timolol belongs to a group of medicines called beta-blockers.
The eye contains a clear, watery liquid that maintains the inside of the eye. This liquid is continuously drained from the eye and new liquid is produced to replace it. If the liquid does not drain quickly enough, the pressure inside the eye increases and, in the long run, your vision could be damaged (a disease called glaucoma). GANFORT works by reducing the production of liquid and also increasing the amount of liquid that is drained. This reduces the pressure inside the eye.
GANFORT eye drops are used to treat high pressure in the eye in adults, including the elderly. This high pressure can cause glaucoma. Your doctor will prescribe GANFORT if other eye drops containing beta-blockers or prostaglandin analogues have not been effective enough on their own.
2. What you need to know before you use GANFORT
Do not use GANFORT eye drops, solution
- if you are allergic to bimatoprost, timolol, beta-blockers, or any of the other ingredients of this medicine (listed in section 6);
- if you currently have or have a history of respiratory problems, such as asthma and/or severe chronic obstructive pulmonary disease (a lung disease that can cause wheezing, difficulty breathing, and/or persistent cough) or other types of respiratory problems.
- if you have heart problems such as slow heart rate, heart block, or heart failure.
Warnings and precautions
Before starting treatment with this medicine, tell your doctor if you have or have had in the past
- coronary heart disease (symptoms may include chest pain or tightness, difficulty breathing, or feeling of suffocation), heart failure, low blood pressure;
- changes in heart rate, such as slow heart rate;
- respiratory problems, asthma, or chronic obstructive pulmonary disease;
- circulatory problems (such as Raynaud's disease or Raynaud's syndrome);
- overactive thyroid gland, as timolol may mask the signs and symptoms of thyroid disease;
- diabetes, as timolol may mask the signs and symptoms of hypoglycemia;
- severe allergic reactions;
- liver or kidney problems;
- problems with the surface of the eye;
- separation of one of the layers of the inside of the eyeball after surgery to reduce eye pressure;
- known risk factors for macular edema (inflammation of the retina that causes vision to worsen), such as cataract surgery.
Tell your doctor before undergoing surgical anesthesia that you are using GANFORT, as timolol may alter the effects of some medicines used during anesthesia.
GANFORT may cause darkening and growth of eyelashes, as well as darkening of the skin around the eyelid. Over time, the color of the iris may also darken. These changes may be permanent and more noticeable if only one eye is being treated. GANFORT may cause hair growth when in contact with the skin surface.
Children and adolescents.
GANFORT should not be used in children and adolescents under 18 years of age.
Other medicines and GANFORT
GANFORT may affect or be affected by other medicines you are using, including other eye drops for the treatment of glaucoma. Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicine. Tell your doctor if you are using or plan to use medicines to lower blood pressure, for the heart, for diabetes treatment, quinidine (used to treat heart conditions and some types of malaria), or for depression treatment, known as fluoxetine and paroxetine.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine. Do not use GANFORT if you are pregnant unless your doctor recommends it.
Do not use GANFORT if you are breastfeeding. Timolol may pass into breast milk. Consult your doctor before using any medicine during breastfeeding.
Driving and using machines
GANFORT may cause blurred vision in some patients. Do not drive or use machines until the symptoms have disappeared.
GANFORT contains benzalkonium chloride
GANFORT contains a preservative called benzalkonium chloride.
This medicine contains 0.15 mg of benzalkonium chloride in every 3 ml of solution, equivalent to 0.05 mg/ml.
Benzalkonium chloride may be absorbed by soft contact lenses and may alter the color of the contact lenses. Remove contact lenses before using this medicine and wait 15 minutes before putting them back in.
Benzalkonium chloride may cause eye irritation, especially if you have dry eye or other corneal diseases (the transparent layer on the front of the eye). Consult your doctor if you feel any unusual sensation, itching, or pain in the eye after using this medicine.
3. How to use GANFORT
Follow the instructions for administration of this medicine exactly as told by your doctor or pharmacist. If you are unsure, consult your doctor or pharmacist again.
The recommended dose is one drop administered once a day, either in the morning or at night, in each eye that needs treatment. Use this medicine every day at the same time.
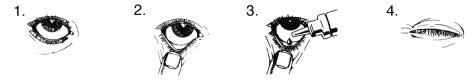
Instructions for use
Do not use the container if the protective seal on the neck of the container is broken before using it for the first time.
5.

- Wash your hands. Tilt your head back and look up at the ceiling.
- Gently pull down the lower eyelid until a small pocket is formed.
- Turn the container upside down and press to release one drop into each eye that needs treatment.
- Release the lower eyelid and close the eye.
- With the eye closed, press the corner of the closed eye (the area near the nose) with your finger and hold for 2 minutes. This helps prevent GANFORT from reaching the rest of the body.
If the drop falls outside the eye, try again.
To prevent contamination, avoid touching the tip of the container to the eye or any other surface. Replace the cap and close the container immediately after use.
If you use GANFORT with another eye medicine, wait at least 5 minutes between administering GANFORT and the other medicine. Use any eye ointment or gel last.
If you use more GANFORT than you should
If you use more GANFORT than you should, it is unlikely to cause you any serious harm. Apply the next dose at the usual time. If you are concerned, talk to your doctor or pharmacist.
If you forget to use GANFORT
If you forget to use GANFORT, apply one drop as soon as you remember and then go back to your usual routine. Do not use a double dose to make up for forgotten doses.
If you stop using GANFORT
GANFORT should be used every day to work properly.
If you have any other questions about using this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. You can usually continue using the eye drops, unless the effects are serious. If you are concerned, talk to a doctor or pharmacist. Do not stop using GANFORT without talking to your doctor.
The following side effects may occur with GANFORT formulations (multi-dose and/or single-dose):
Very common side effects
May affect more than 1 in 10 people
Affecting the eye
redness.
Common side effects
May affect up to 1 in 10 people
Affecting the eye
burning, itching, stinging, conjunctival irritation (the transparent layer of the eye), sensitivity to light, eye pain, sticky eyes, dryness of the eyes, feeling of something in the eye, small erosions on the surface of the eye with or without inflammation, difficulty seeing clearly, redness and itching of the eyelids, hair growth around the eye, darkening of the eyelids, darker color around the eyes, headache, longer eyelashes, eye irritation, watery eyes, inflamed eyelids, reduced vision.
Affecting other parts of the body
runny nose, headache.
Uncommon side effects
May affect up to 1 in 100 people
Affecting the eye
abnormal sensation in the eye, inflammation of the iris, conjunctivitis (inflammation of the transparent layer of the eye), painful eyelids, tired eyes, ingrown eyelashes, darkening of the iris color, sunken eyes, drooping eyelid, retracted eyelid (moving away from the eye surface, causing incomplete closure of the eyelids), tightness of the eyelid skin, darkening of the eyelashes.
Affecting other parts of the body
shortness of breath.
Frequency not known
Affecting the eye
cystoid macular edema (inflammation of the retina that causes vision to worsen), eye inflammation, blurred vision, eye discomfort.
Affecting other parts of the body
difficulty breathing/wheezing (production of sounds when breathing), symptoms of allergic reaction (swelling, redness of the eye, and skin rash), changes in taste, dizziness, slow heart rate, high blood pressure, sleep disturbances, nightmares, asthma, hair loss, skin discoloration (around the eye), fatigue.
As the following additional side effects have been seen in patients using timolol or bimatoprost eye drops, it is possible that they may occur with GANFORT. Like other eye medicines, timolol is absorbed into the bloodstream. This may cause side effects similar to those seen with beta-blockers given by injection or orally. The likelihood of side effects with eye drop use is less than with oral or injected medicines. Among the side effects listed are those observed with bimatoprost and timolol when used to treat eye conditions:
- Severe allergic reactions with inflammation and difficulty breathing that can be life-threatening.
- Hypoglycemia.
- Depression; memory loss; hallucination.
- Fainting; stroke; reduced blood flow to the brain; worsening of myasthenia gravis (increased muscle weakness); tingling sensation.
- Decreased sensitivity on the eye surface; double vision; drooping eyelid; separation of one of the layers of the eyeball after surgery to reduce eye pressure; inflammation of the eye surface, bleeding in the back of the eye (retinal hemorrhage), inflammation inside the eye, increased blinking.
- Heart failure; irregular heartbeat or interruption of heartbeat; slow or fast heart rate, excess fluid, mainly water, that accumulates in the body; chest pain.
- Low blood pressure; swelling or coldness of the hands, feet, and limbs, caused by constriction of blood vessels.
- Cough, worsening of asthma, worsening of chronic obstructive pulmonary disease (COPD).
- Diarrhea; stomach pain; nausea and vomiting; indigestion; dry mouth.
- Scaly red patches on the skin; skin rash.
- Muscle pain.
- Decreased sex drive, sexual dysfunction.
- Weakness.
- Increased results of some blood tests that indicate how the liver is working.
Other side effects reported with phosphate-containing eye drops
This medicine contains 2.85 mg of phosphates in every 3 ml of solution, equivalent to 0.95 mg/ml. If you have severe damage to the transparent layer on the front of the eye (cornea), treatment with phosphates, in very rare cases, may cause cloudy patches on the cornea due to calcium.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if it is possible side effects not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of GANFORT
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the container after EXP. The expiry date is the last day of the month shown.
This medicine does not require any special storage conditions.
Once opened, the solutions may become contaminated, which can cause eye infections. Therefore, you should discard the container 4 weeks after first opening, even if there is still some solution left. To remind you, write the date you opened the container on the space provided on the carton.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Package Contents and Additional Information
GANFORT Composition
- The active ingredients are bimatoprost 0.3 mg/ml and timolol 5 mg/ml, corresponding to timolol maleate 6.8 mg/ml.
- The other ingredients are benzalkonium chloride (preservative), sodium chloride, sodium hydrogen phosphate heptahydrate, citric acid monohydrate, and purified water. Small amounts of hydrochloric acid or sodium hydroxide may be added to adjust the solution to the correct pH level.
Product Appearance and Package Contents
GANFORT is a colorless or slightly yellowish, transparent eye drop solution in a plastic container. Each pack contains 1 plastic container or 3 plastic containers, each with a screw cap. Each container holds 3 milliliters of solution, filling it up to about half. The content is sufficient for 4 weeks of treatment. Only certain package sizes may be marketed.
Marketing Authorization Holder and Manufacturer
AbbVie Deutschland GmbH & Co. KG
Knollstraße
67061 Ludwigshafen
Germany
You can request more information about this medication by contacting the local representative of the marketing authorization holder.
Belgium/Belgique/Belgien/Luxembourg/Luxemburg/Netherlands Allergan n.v. Tel: +32 (0)2 351 24 24 | Iceland Teva Pharma Iceland ehf. Phone: +354 550 3300 |
Bulgaria Allergan Bulgaria EOOD Phone: +359 (0) 800 20 280 | Italy Allergan S.p.A. Tel: +39 06 509 562 90 |
Czech Republic Allergan CZ s.r.o. Tel: +420 800 188 818 | Latvia AbbVie SIA Tel: +371 676 05000 Lithuania AbbVie UAB Tel: +370 5 205 3023 |
Denmark/Norway/Finland/Sweden Allergan Norden AB Phone: +45 80 88 45 60 (DK); +47 80 01 04 97 (NO); +358 800 115 003 (FI); +46 (0)8 594 100 00 (SE) | Hungary Allergan Hungary Kft. Tel: +36 80 100 101 |
Germany Pharm-Allergan GmbH Tel: +49 69 92038 10 50 | Austria Pharm-Allergan GmbH Tel: +43 1 99460 6355 |
Estonia AbbVie OÜ Tel: +372 6231011 | Poland Allergan Sp. z o.o. Tel: +48 22 256 3700 |
Greece/Cyprus Allergan Hellas Pharmaceuticals S.A. Phone: +30 210 74 73 300 | Portugal Profarin Lda. Tel: +351 21 425 3242 |
Spain AbbVie Spain, S.L.U. Tel: +34 913840910 | Romania Allergan S.R.L. Tel: +40 21 301 53 02 |
France Allergan France SAS Tel: +33 (0)1 49 07 83 00 | Slovenia Ewopharma d.o.o. Tel: +386 (0) 590 848 40 |
Croatia Ewopharma d.o.o. Tel: +385 1 6646 563 | Slovak Republic Allergan SK s.r.o. Tel: +421 800 221 223 |
Ireland/Malta Allergan Pharmaceuticals Ireland Tel: 1800 931 787 (IE); +356 27780331 (MT) | United Kingdom Allergan Ltd. Tel: +44 (0)1628 494026 |
Date of Last Revision of this Leaflet:<{MM/YYYY}><{Month YYYY}>
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu/.
- Country of registration
- Average pharmacy price8.46 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to GANFORT 0.3 mg/mL + 5 mg/mL Eye Drops, SolutionDosage form: EYEDROP, 10 mg/ml + 5 mg/mlActive substance: timolol, combinationsManufacturer: Novartis Europharm LimitedPrescription requiredDosage form: EYEDROP, 0.3 mg/ml + 5 mg/mlActive substance: timolol, combinationsManufacturer: Brill Pharma S.L.Prescription requiredDosage form: EYE DROP, 0.3 mg/ml + 5 mg/mlActive substance: timolol, combinationsManufacturer: Laboratorio Stada S.L.Prescription required
Online doctors for GANFORT 0.3 mg/mL + 5 mg/mL Eye Drops, Solution
Discuss questions about GANFORT 0.3 mg/mL + 5 mg/mL Eye Drops, Solution, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















