
FLUIDASA 5 mg/ml ORAL SOLUTION

How to use FLUIDASA 5 mg/ml ORAL SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
PACKAGE LEAFLET: INFORMATION FOR THE USER
FLUIDASA 5 mg/ml oral solution
Mepifilina (mepiramine acefyllinate)
Read all of this leaflet carefully before you start taking this medicine.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you, do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any of the side effects, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
Contents of the pack:
- What Fluidasa Solution is and what it is used for
- Before taking Fluidasa Solution
- How to take Fluidasa Solution
- Possible side effects
- Storage of Fluidasa Solution
- Further information
1. What FLUIDASA SOLUTION is and what it is used for
This medicinal product contains mepifilina, which belongs to the group of antihistamine medicines.
Fluidasa Solution is used for the treatment of bronchoconstriction in patients with acute and chronic bronchitis (inflammation of the bronchi).
2. Before taking FLUIDASA SOLUTION
Do not take Fluidasa Solution:
- If you are allergic (hypersensitive) to mepifilina or any of the other ingredients of Fluidasa Solution.
- If you have any liver disease.
- If you have urinary bladder obstruction.
- If you have symptomatic prostatic hypertrophy or urinary retention.
- If you have narrow-angle glaucoma.
- If you have fructose intolerance because this medicinal product contains a sugar called sorbitol.
Be cautious when taking Fluidasa Solution:
- If you are sensitive to antihistamines (allergy medicines) as patients sensitive to one antihistamine may be sensitive to others.
- If you are an elderly patient as you are more likely to experience side effects such as dizziness, sedation, confusion, low blood pressure, dry mouth, or urinary retention. If these effects persist or are severe, you should inform your doctor in case the medication needs to be discontinued.
- It is not recommended for use in newborns or premature infants due to the increased sensitivity to some effects of the medicinal product, such as anticholinergic effects (e.g., central nervous system stimulation) and a greater tendency to convulsions.
Use of other medicines:
Tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including those obtained without a prescription.
Especially, inform your doctor if you are taking the following medicines as some of their effects may be increased:
- Medicines belonging to the group of anticholinergic medicines (e.g., some medicines used for relief of spasms or contractions of the stomach, intestine, or bladder)
- Medicines used for the treatment of depression, such as monoamine oxidase inhibitors (MAOIs), tricyclic antidepressants, or maprotiline.
- If skin allergy tests need to be performed, it is recommended to discontinue the administration of Fluidasa Solution at least 72 hours before starting the test as it may give false-negative results (negative results that are actually positive).
Taking Fluidasa Solution with food and drinks:
You should avoid consuming alcoholic beverages during the administration of this medicinal product as it may increase the effects of alcohol.
Pregnancy and breastfeeding
It is not recommended to take Fluidasa Solution during pregnancy.
It is not recommended to take Fluidasa Solution during breastfeeding.
Consult your doctor or pharmacist before using any medicine.
Driving and using machines:
Fluidasa Solution may cause drowsiness, affecting mental and/or physical ability. If you experience these effects, avoid driving vehicles or using machines.
Important information about some of the ingredients of Fluidasa Solution
This medicinal product contains sorbitol. If your doctor has told you that you have an intolerance to some sugars, consult with them before taking this medicinal product. It may have a mild laxative effect as it contains 4 g of sorbitol per 15 ml dose.
Caloric value: 2.6 kcal/g of sorbitol.
It may cause allergic reactions (possibly delayed) as it contains methyl parahydroxybenzoate (E-218) and propyl parahydroxybenzoate (E-216).
3. How to take FLUIDASA SOLUTION
Fluidasa Solution is administered orally.
Follow exactly the administration instructions of Fluidasa Solution as indicated by your doctor.
Consult your doctor or pharmacist if you have any doubts.
1 dose of 5 ml contains approximately 25 mg of mepifilina, and 1 dose of 15 ml contains 75 mg of mepifilina.
The average normal dose is 8 mg per kg of body weight and day, divided into 4 doses. The maximum dose is 18 mg per kg of body weight and day, divided into 4 doses.
The normal dose, as a guideline and in relation to body weight, is as follows:
- Children under 5 years: 5 to 10 ml of the solution, 3 or 4 times a day.
- Children over 5 years: 10 to 15 ml of the solution, 3 or 4 times a day.
- Adults: 15 to 30 ml (one or two 15 ml doses) of the solution, 4 times a day.
Instructions for correct administration
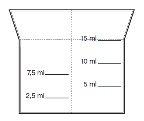
Take the amount of medicine to be taken using the measuring cup that has the following measures marked: 2.5 ml, 5 ml, 7.5 ml, 10 ml, and 15 ml. Fill it with the solution up to the line indicating your dose. After use, wash the measuring cup with water.
In infants, it is recommended to use Fluidasa Drops.
If you feel that the effect of Fluidasa Solution is too strong or too weak, consult your doctor or pharmacist.
If you take more Fluidasa Solution than you should:
If you have taken more Fluidasa Solution than you should, consult your doctor or pharmacist immediately.
In cases of overdose, symptoms of anticholinergic effects such as clumsiness, instability, intense sleep, dry mouth, nose, or throat, flushing, or redness, variations in the normal heart rate, central nervous system depression (hallucinations), or low blood pressure may appear. In children, anticholinergic stimulant effects on the CNS are more likely.
In case of overdose or accidental ingestion, consult the Toxicology Information Service, phone: 91 562 04 20.
If you forget to take Fluidasa Solution:
Do not take a double dose to make up for forgotten doses.
Take your dose as soon as possible and continue taking it every day at the time indicated by your doctor.
4. Possible side effects
Like all medicines, Fluidasa Solution can cause side effects, although not everybody gets them.
The side effects found have been:
Very common (at least 1 in 10 patients): drowsiness.
Common (at least 1 in 100 patients): blurred vision, confusion, difficulty urinating, dizziness, dry mouth, rapid heart rate, ringing in the ears, skin rash, discomfort or pain in the stomach.
Uncommon (at least 1 in 1000 patients): abnormal blood conditions (blood dyscrasias) and variations in the normal heart rate.
If you experience any side effect that you think is serious or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
Reporting of side effects
If you experience any side effects, consult your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. You can also report them directly through the Spanish Medicines and Health Products Agency's website: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicinal product.
5. Storage of FLUIDASA SOLUTION
Keep out of the reach and sight of children.
Do not store above 25°C.
Do not use Fluidasa solution after the expiry date stated on the packaging after CAD. The expiry date is the last day of the month indicated.
Medicines should not be disposed of via wastewater or household waste. Place the packaging and any unused medicines in the SIGRE collection point at your pharmacy. If you have any further questions, ask your pharmacist how to dispose of the packaging and any unused medicines. This will help protect the environment.
6. Further information
Composition of Fluidasa solution
- The active ingredient is: mepifilina (mepiramine acefyllinate). Each ml of solution contains 5 mg of mepifilina.
- The other ingredients are: sodium saccharin, sorbitol (E-420), methyl parahydroxybenzoate (E-218), propyl parahydroxybenzoate (E-216), vanilla flavor, and purified water.
Appearance and packaging of the product
Topaz glass bottle containing 250 ml of oral solution accompanied by a 15 ml measuring cup
Marketing authorization holder and manufacturer:
Marketing authorization holder:
Teofarma, S.r.l.
Via F. Lli Cervi, 8
27010 Valle Salimbene (PV) – Italy
Manufacturer:
Teofarma S.r.l.
Viale Certosa, 8/A
27100 Pavia – Italy
Other presentations
Fluidasa Drops. Bottle of 30 ml oral solution and dropper cap.
Fluidasa Capsules. Package of 20 capsules.
Fluidasa Injectable. Package containing 10 ampoules of 5 ml.
This leaflet was approved in July 2022
Detailed and updated information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price4.21 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to FLUIDASA 5 mg/ml ORAL SOLUTIONDosage form: CAPSULE, 150 mgActive substance: mepyramine theophyllinacetateManufacturer: Teofarma S.R.L.Prescription requiredDosage form: ORAL SOLUTION/SUSPENSION DROPS, 20 mg/mlActive substance: mepyramine theophyllinacetateManufacturer: Teofarma S.R.L.Prescription requiredDosage form: INJECTABLE, -Active substance: diprophylline, combinationsManufacturer: Ionfarma S.L.Prescription required
Online doctors for FLUIDASA 5 mg/ml ORAL SOLUTION
Discuss questions about FLUIDASA 5 mg/ml ORAL SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















