
DROPERIDOL KALCEKS 1.25 mg/mL INJECTABLE SOLUTION

How to use DROPERIDOL KALCEKS 1.25 mg/mL INJECTABLE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Droperidol Kalceks 1.25 mg/ml Solution for Injection
droperidol
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or nurse.
- If you experience any side effects, talk to your doctor or nurse, even if they are not listed in this leaflet. See section 4.
Contents of the Package Leaflet
- What is Droperidol Kalceks and what is it used for
- What you need to know before you are given Droperidol Kalceks
- How Droperidol Kalceks will be given to you
- Possible side effects
- Storage of Droperidol Kalceks
- Contents of the pack and further information
1. What is Droperidol Kalceks and what is it used for
Droperidol Kalceks is a solution for injection that contains droperidol, an active substance belonging to the group of medicines called butyrophenone derivatives. Droperidol is used to prevent the feeling of nausea or vomiting when waking up after an operation or when receiving morphine-derived analgesics after an operation.
2. What you need to know before you are given Droperidol Kalceks
Droperidol Kalceks must not be given to you
- if you are allergic to droperidol or any of the other ingredients of this medicine (listed in section 6);
- if you are allergic to a group of medicines used to treat psychiatric disorders, called butyrophenones (e.g. haloperidol, triperidol, benperidol, melperone, domperidone);
- if you or someone in your family has heart rhythm disorders (ECG);
- if you have low levels of potassium or magnesium in your blood;
- if your heart rate is less than 55 beats per minute (your doctor or nurse will check this), or you are taking medicines that may cause this situation;
- if you have a tumor of the adrenal glands (pheochromocytoma);
- if you are in a coma;
- if you have Parkinson's disease;
- if you have severe depression.
Warnings and precautions
Talk to your doctor or nurse before you are given this medicine, as special precautions are required:
- if you have epilepsy or a history of epilepsy;
- if you have heart problems or a history of heart disease;
- if you have a family history of sudden death;
- if you have kidney problems (especially if you are on long-term dialysis);
- if you have any lung disease or breathing difficulties;
- if you have prolonged vomiting or diarrhea;
- if you are using insulin;
- if you are taking diuretics that eliminate potassium (e.g. furosemide or bendroflumethiazide);
- if you are taking laxatives;
- if you are taking glucocorticoids (a type of steroid hormone);
- if you or someone in your family has a history of blood clots, as this type of medicine has been associated with the formation of blood clots;
- if you are or have been a heavy drinker (of alcohol).
Other medicines and Droperidol Kalceks
Tell your doctor or nurse if you are taking, have recently taken, or might take any other medicines, as some medicines cannot be mixed with droperidol.
You must not be giventhis medicine if you are taking any of the following medicines, as the combination increases the risk of irregular heartbeats that could lead to a heart attack (myocardial infarction):
What the medicine is used for | Medicine(s) |
Heart rhythm disorders, irregular heartbeats | Class IA and III antiarrhythmics |
Bacterial infections | Macrolide and fluoroquinolone antibiotics |
Malaria | Antimalarials |
Allergies | Antihistamines |
Mental illnesses, e.g. schizophrenia | Antipsychotics |
Heartburn | Cisapride |
Parasite infestation or fungal infection | Pentamidine |
Nausea or vomiting | Domperidone |
Opiate dependence; pain | Methadone |
Metoclopramide and other neuroleptics should be avoided while using droperidol, as the risk of movement disorders induced by these medicines is increased.
Other medicines that may affect or be affected when used simultaneously with droperidol.
The droperidol, the active substance of this medicine:
- may increase the effects of sedatives such as barbiturates, benzodiazepines, and morphine-derived products;
- may increase the effects of medicines used to lower blood pressure;
- may increase the effects of various medicines, such as certain antifungals, antivirals, and antibiotics.
If you are taking any of these medicines, you should inform your doctor or nurse.
Droperidol Kalceks with alcohol
Avoid drinking alcohol during the 24 hours before and after using droperidol.
Pregnancy, breastfeeding, and fertility
If you are pregnant, consult your doctor to decide if you should receive this medicine.
In newborns of mothers who have received droperidol in the last trimester (last three months of pregnancy), the following symptoms may appear: tremors, muscle stiffness and/or weakness, drowsiness, agitation, respiratory problems, and difficulty feeding. If your child has any of these symptoms, you may need to consult your doctor.
If you are breastfeeding and are going to receive droperidol, the treatment will be reduced to a single administration. You will be able to breastfeed again after waking up after the operation.
Ask your doctor for advice before taking any medicine.
Driving and using machines
The influence of droperidol on the ability to drive and use machines is significant.
Do not drive or use machines for at least 24 hours after taking this medicine.
Droperidol Kalceks contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per ml, which is essentially "sodium-free".
3. How Droperidol Kalceks will be given to you
This medicine will be given to you by a doctor or nurse as an injection into a vein.
The dose of droperidol and the way it is given will depend on the situation. Your doctor will determine the amount of medicine you need based on different criteria, such as your weight, age, and medical condition.
If you have any further questions about the use of this medicine, ask your doctor or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Tell your doctor or nurse immediatelyif you experience any of the following serious side effects:
- Increased body temperature, sweating, salivation, muscle stiffness, or tremors. These may be symptoms of a condition called malignant neuroleptic syndrome (a rare side effect).
- Severe allergic reaction or rapid swelling of the face or throat; difficulty swallowing; urticaria and difficulty breathing (a rare side effect).
The following side effects have been reported:
Common (may affect less than 1 in 10 people):
- Drowsiness
- Low blood pressure
Uncommon (may affect less than 1 in 100 people):
- Anxiety
- Rolling of the eyes
- Fast heart rate, e.g. more than 100 beats per minute
- Dizziness
Rare (may affect up to 1 in 1,000 people)
- Confusion
- Agitation
- Irregular heartbeats
- Rash
Very rare (may affect less than 1 in 10,000 people)
- Blood disorders (usually diseases related to red blood cells or platelets). Your doctor will advise you
- Change in mood to sadness, anxiety, depression, and irritability
- Involuntary muscle movements
- Seizures or tremors
- Heart attack (cardiac arrest)
- Torsade de pointes(a life-threatening irregular heartbeat)
- Prolongation of the QT interval in the electrocardiogram (ECG) (a disease that affects the heartbeat)
- Sudden death
Frequency not known (cannot be estimated from the available data)
- Inadequate secretion of antidiuretic hormone (too much of the hormone is released, leading to excess water and decreased sodium levels in the body)
- Hallucinations
- Seizures
- Parkinson's disease
- Fainting
- Breathing difficulties
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist, even if they are not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaRAM.es By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Droperidol Kalceks
Keep this medicine out of the sight and reach of children.
This medicine does not require any special storage temperature.
Keep the ampoules in the outer packaging to protect them from light.
Shelf life after opening of the ampoule
Use immediately after first opening.
Shelf life after dilution
Compatibility and stability of Droperidol Kalceks 1.25 mg/ml solution for injection with morphine in a 9 mg/ml (0.9%) sodium chloride injection solution in polypropylene (PP) and polycarbonate (PC) syringes have been demonstrated for 14 days at 25°C (protected from light) and at temperatures between 2 and 8°C.
From a microbiological point of view, the diluted solution should be used immediately. If not used immediately, the in-use storage times and conditions are the responsibility of the user and normally should not exceed 24 hours at a temperature of 2 to 8°C, unless the dilution has been made in controlled and validated aseptic conditions.
Do not use this medicine after the expiry date which is stated on the carton and ampoule after ‘EXP’. The expiry date is the last day of the month stated.
For single use only. Any unused solution should be discarded.
The solution should be inspected visually before use. Do not use this medicine if you notice visible signs of deterioration. It should only be used if the solution is clear, colorless, and free of visible particles.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and further information
Droperidol Kalceks Composition
- The active substance is droperidol.
Each 1 ml ampoule of solution for injection contains 1.25 mg of droperidol.
The other ingredients are: tartaric acid, mannitol, sodium hydroxide (for pH adjustment), water for injections.
Appearance of Droperidol Kalceks and pack contents
Clear and colorless solution, free of visible particles.
Ampoules of type I amber glass containing 1 ml of solution for injection with a break point. The ampoules are placed in a tray and packaged in a cardboard box.
Pack sizes: 5 or 10 ampoules.
Not all pack sizes may be marketed.
Marketing authorisation holder and manufacturer:
AS KALCEKS
Krustpils iela 71E, Riga, LV-1057, Latvia
Tel.: +371 67083320
E-mail: [email protected]
You can obtain further information on this medicine from the local representative of the marketing authorisation holder:
Kern Pharma, S.L.
Venus, 72 - Pol. Ind. Colón II
08228 Terrassa – Barcelona
Spain
Tel: +34 93 700 25 25
Date of last revision of this leaflet:March 2021
Detailed information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) (http://www.aemps.gob.es/)
------------------------------------------------------------------------------------------------------------------
This information is intended only for healthcare professionals:
Incompatibilities
Incompatible with barbiturates. This medicine should not be mixed with other medicines, except those mentioned below in the "Instructions for use" section.
Instructions for use
For single use only. The unused solution should be discarded.
The solution should be inspected visually before use. Do not use this medicine if you notice visible signs of deterioration. It should only be used if the solution is clear, colorless, and free of visible particles.
For use in Patient-Controlled Analgesia (PCA): withdraw the droperidol and morphine with a syringe and prepare the desired volume with a 9 mg/ml (0.9%) sodium chloride injection solution.
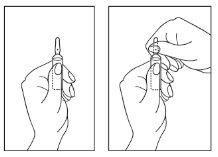
Instructions for opening the ampoule:
- Turn the ampoule with the colored point upwards. If some solution remains in the top part of the ampoule, gently tap it with your finger to make the solution flow down to the bottom part of the ampoule.
- Use both hands to open it and, while holding the bottom part of the ampoule with one hand, use the other hand to break the top part of the ampoule in the opposite direction of the colored point (see the images below).
The disposal of unused medicine and all materials that have come into contact with it will be carried out in accordance with local regulations.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to DROPERIDOL KALCEKS 1.25 mg/mL INJECTABLE SOLUTIONDosage form: INJECTABLE, 2.5 mg/mlActive substance: droperidolManufacturer: Hikma Farmaceutica (Portugal) S.A.Prescription requiredDosage form: INJECTABLE, 2.5 mg/1 mLActive substance: droperidolManufacturer: SubstipharmPrescription requiredDosage form: Solution for injection, 2.5 mg/mLActive substance: droperidolManufacturer: Kalceks AsPrescription required
Online doctors for DROPERIDOL KALCEKS 1.25 mg/mL INJECTABLE SOLUTION
Discuss questions about DROPERIDOL KALCEKS 1.25 mg/mL INJECTABLE SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions











