
COVERSORAL 5 mg ORALLY DISPERSIBLE TABLETS

How to use COVERSORAL 5 mg ORALLY DISPERSIBLE TABLETS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Coversoral 5mg orodispersible tablets
perindopril arginine
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack and other information
- What is Coversoral and what is it used for
- What you need to know before you take Coversoral
- How to take Coversoral
- Possible side effects
- Storing Coversoral
- Contents of the pack and other information
1. What is Coversoral and what is it used for
Coversoral is an angiotensin-converting enzyme inhibitor (ACE inhibitor). It works by widening the blood vessels, making it easier for your heart to pump blood through them.
Coversoral is used to:
- treat high blood pressure (hypertension),
- treat heart failure (a condition where the heart is unable to pump enough blood to meet the body's needs),
- reduce the risk of heart events, such as heart attacks, in patients with stable coronary artery disease (a condition where the blood supply to the heart is reduced or blocked) who have already had a heart attack and/or undergone surgery to improve blood flow to the heart by widening the blood vessels that supply it.
2. What you need to know before you take Coversoral
Do not take Coversoral
- if you are allergic to perindopril, or any of the other ingredients of this medicine (listed in section 6), or to any other ACE inhibitor,
- if you have experienced symptoms such as wheezing, swelling of the face, lips, tongue, or throat, intense itching, or severe skin rashes with previous treatment with ACE inhibitors, or if you or a family member have had these symptoms in any other circumstances (a condition known as angioedema),
- if you are more than 3 months pregnant (it is also better to avoid Coversoral at the start of pregnancy – see section Pregnancy and breast-feeding),
- if you have diabetes or kidney problems and are being treated with a blood pressure-lowering medicine that contains aliskiren,
- if you are having dialysis or any other type of blood filtration. Depending on the machine used, Coversoral may not be suitable for you,
- if you have kidney problems where the blood flow to your kidneys is reduced (renal artery stenosis),
- if you have taken or are currently taking sacubitril/valsartan, a medicine for heart failure, as this increases the risk of angioedema (rapid swelling under the skin in an area such as the throat) (see “Warnings and precautions” and “Using Coversoral with other medicines”).
Warnings and precautions
If any of the following apply to you, tell your doctor, pharmacist, or nurse before taking Coversoral, if you:
- have aortic stenosis (narrowing of the main blood vessel that leaves the heart) or hypertrophic cardiomyopathy (a disease of the heart muscle) or renal artery stenosis (narrowing of the artery that supplies blood to the kidneys),
- have any other heart problems,
- have liver problems,
- have kidney problems or are on dialysis,
- have abnormally high levels of a hormone called aldosterone in your blood (primary aldosteronism),
- suffer from a collagen vascular disease (a disease of the connective tissue) such as systemic lupus erythematosus or scleroderma,
have diabetes,
- are on a low-salt diet or use salt substitutes that contain potassium,
- are going to have an anaesthetic and/or major surgery,
- are going to have an LDL apheresis (a procedure to remove cholesterol from your blood
using a machine),
- are going to receive a treatment to make you less sensitive to the effects of an allergy to bee or wasp stings,
have recently had diarrhoea or vomiting, or are dehydrated,
- have been told by your doctor that you have an intolerance to some sugars,
- have been told by your doctor that you have phenylketonuria,
- are of black origin, as you may have a higher risk of angioedema and this medicine may be less effective at lowering your blood pressure than in patients who are not of black origin,
- are taking any of the following medicines used to treat high blood pressure (hypertension):
- an angiotensin II receptor antagonist (ARA) (also known as "sartans" - e.g. valsartan, telmisartan, irbesartan), especially if you have kidney problems related to diabetes.
- aliskiren.
Your doctor may monitor your kidney function, blood pressure, and blood electrolyte levels (e.g. potassium) at regular intervals.
See also the information under the heading “Do not take Coversoral”.
- racecadotril (used to treat diarrhoea)
- sirolimus, everolimus, temsirolimus, and other medicines belonging to the class called mTor inhibitors (used to prevent transplant rejection and to treat cancer),
- sacubitril (available as a fixed-dose combination with valsartan), used to treat long-term heart failure,
- linagliptin, saxagliptin, sitagliptin, vildagliptin, and other medicines belonging to the class of gliptins (used to treat diabetes).
Angioedema
In patients treated with ACE inhibitors, including Coversoral, angioedema (a severe allergic reaction with swelling of the face, lips, tongue, or throat with difficulty swallowing or breathing) has been reported. This can occur at any time during treatment. If you develop these symptoms, you should stop taking Coversoral and see a doctor immediately. See also Section 4.
You should tell your doctor if you think you are (or might become) pregnant. Coversoral is not recommended at the start of pregnancy, and you should not take it if you are more than 3 months pregnant, as it may cause serious harm to your baby if taken after the third month of pregnancy.
Children and adolescents
Coversoral is not recommended for use in children and adolescents.
Using Coversoral with other medicines
Tell your doctor or pharmacist if you are using or have recently used or might use any other medicines.
Treatment with Coversoral may be affected by other medicines. Your doctor may need to change your dose and/or take other precautions. These include:
- other medicines for high blood pressure, including angiotensin II receptor antagonists (ARAs), aliskiren (see also the information under the headings “Do not take Coversoral” and “Warnings and precautions”), or diuretics (medicines that increase the amount of urine produced by the kidneys),
- potassium-sparing medicines (e.g. triamterene, amiloride), potassium supplements, or salt substitutes that contain potassium, other medicines that may increase potassium levels in your body (such as heparin, a medicine used to thin the blood and prevent clots; trimethoprim and cotrimoxazole, also known as trimethoprim/sulfamethoxazole, for bacterial infections),
- potassium-sparing medicines used in the treatment of heart failure: eplerenone and spironolactone at doses between 12.5 mg and 50 mg per day,
- lithium for mania or depression,
- non-steroidal anti-inflammatory medicines (e.g. ibuprofen) for pain relief or high doses of acetylsalicylic acid, a substance found in many medicines used to relieve pain and reduce fever, as well as to prevent blood clotting,
- medicines for diabetes (such as insulin or metformin),
- baclofen (used to treat muscle stiffness in diseases such as multiple sclerosis),
- medicines for mental disorders such as depression, anxiety, schizophrenia, etc. (e.g. tricyclic antidepressants, antipsychotics),
immunosuppressants (medicines that reduce the body's defence mechanism) used
- to treat autoimmune disorders or after a transplant (e.g. cyclosporin, tacrolimus),
- trimethoprim (for the treatment of infections),
- estramustine (used in the treatment of cancer),
- medicines that are commonly used to treat diarrhoea (racecadotril) or to prevent transplant rejection (sirolimus, everolimus, temsirolimus, and other medicines belonging to the class called mTor inhibitors). See section “Warnings and precautions”,
- sacubitril/valsartan (used to treat long-term heart failure). See sections “Do not take Coversoral” and “Warnings and precautions”,
- allopurinol (for the treatment of gout),
- procainamide (for the treatment of irregular heartbeats),
- vasodilators, including nitrates (medicines that cause the blood vessels to widen),
medicines used to treat low blood pressure, shock, or asthma (e.g. ephedrine, noradrenaline, or adrenaline),
- gold salts, especially when given intravenously (used to treat rheumatoid arthritis).
Taking Coversoral with food and drink
It is preferable to take Coversoral before a meal.
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before taking any medicine.
Pregnancy
You should tell your doctor if you think you are (or might become) pregnant. Normally, your doctor will advise you to stop taking Coversoral before you become pregnant or as soon as you know you are pregnant and will recommend that you take another medicine instead of Coversoral. Coversoral is not recommended at the start of pregnancy, and you must not take it when you are more than 3 months pregnant, as it may cause serious harm to your baby if taken after the third month of pregnancy.
Breast-feeding
Tell your doctor if you are breast-feeding or about to start breast-feeding. Coversoral is not recommended for mothers who are breast-feeding, and your doctor may choose another treatment for you if you want to continue breast-feeding, especially if your baby is newborn or was born prematurely.
Driving and using machines
Coversoral does not affect alertness, but due to the blood pressure-lowering effect, some patients may experience dizziness or weakness. If this happens to you, your ability to drive or use machines may be impaired.
Coversoral contains lactose and aspartame.
If your doctor has told you that you have an intolerance to some sugars, consult them before taking this medicine.
This medicine contains 0.2 mg of aspartame in each tablet. Aspartame is a source of phenylalanine, which may be harmful if you have phenylketonuria (PKU), a rare genetic disorder in which phenylalanine builds up because the body cannot remove it properly.
3. How to take Coversoral
Take Coversoral exactly as your doctor or pharmacist has told you. If you are not sure, ask your doctor or pharmacist.
Place the tablet on your tongue to disintegrate and swallow it with your saliva, preferably at the same time each day, in the morning, before breakfast.
Your doctor will decide the correct dose for you.
The recommended doses are as follows:
High blood pressure: The initial and maintenance dose is usually 5 mg once daily. If necessary, after one month of treatment, your doctor may increase the dose to 10 mg once daily. The maximum recommended dose for high blood pressure is 10 mg once daily.
If you are 65 years or older, the initial dose is usually 2.5 mg once daily. If necessary, after one month of treatment, your doctor may increase the dose to 5 mg once daily, and if necessary, up to 10 mg once daily.
Heart failure: The initial dose is usually 2.5 mg once daily. If necessary, after two weeks of treatment, your doctor may increase the dose to 5 mg once daily, which is the maximum recommended dose for heart failure.
Stable coronary artery disease: The initial dose is usually 5 mg once daily. If necessary, after two weeks of treatment, your doctor may increase the dose to 10 mg once daily, which is the maximum recommended dose for this indication.
If you are 65 years or older, the initial dose is usually 2.5 mg once daily. If necessary, after one week of treatment, your doctor may increase the dose to 5 mg once daily, and if necessary, one week later, the dose may be increased to 10 mg once daily.
If you take more Coversoral than you should
If you have taken more Coversoral 5 mg tablets than you should, contact your doctor or call the Poisons Information Service, telephone 91-562 04 20, immediately.
The most common symptom in case of overdose is low blood pressure with possible symptoms of dizziness or fainting. If this happens, lying down with your legs raised may help.
Use in children and adolescents
Coversoral is not recommended for use in children and adolescents.
If you forget to take Coversoral
It is important to take this medicine every day as continuous treatment is more effective. However, if you forget to take a dose of Coversoral, take the next dose at the usual time. Do not take a double dose to make up for forgotten doses.
If you stop taking Coversoral
As treatment with Coversoral will usually be long-term, you should talk to your doctor before stopping this medicine.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible Adverse Effects
Like all medicines, this medicine can have adverse effects, although not all people suffer from them.
Stop treatment with this medicine and go immediately to your doctor if you experience any of the following adverse effects that may be serious:
- inflammation of the face, lips, mouth, tongue, or throat, difficulty breathing (angioedema) (See Section 2 "Warnings and Precautions") (Uncommon - may affect up to 1 in 100 people),
- severe dizziness or fainting due to low blood pressure (Common - may affect up to 1 in 10 people),
- abnormally fast or irregular heartbeats, chest pain (angina) or heart attack (Very Rare - may affect up to 1 in 10,000 people),
- weakness in arms or legs, or speech problems that could be a sign of a possible stroke (Very Rare - may affect up to 1 in 10,000 people),
- sudden wheezing, chest pain, shortness of breath, or difficulty breathing (bronchospasm) (Uncommon - may affect up to 1 in 100 people),
- inflammation of the pancreas that can cause severe abdominal and back pain accompanied by discomfort (Very Rare - may affect up to 1 in 10,000 people),
- yellowing of the skin or eyes (jaundice) that could be a sign of hepatitis (Very Rare - may affect up to 1 in 10,000 people),
- skin rash that often starts with red spots that itch on the face, arms, or legs (erythema multiforme) (Very Rare - may affect up to 1 in 10,000 people).
Tell your doctor if you experience any of the following adverse effects:
- Common (may affect up to 1 in 10 people):
- headache,
- dizziness,
- vertigo,
- tingling,
- visual disturbances,
- tinnitus (ringing in the ears),
- cough,
- breathing difficulties (dyspnea),
- digestive disorders (nausea, vomiting, abdominal pain, altered taste, dyspepsia, or difficulty digesting, diarrhea, constipation),
- allergic reactions (such as skin rashes, itching),
- muscle cramps,
- feeling of weakness.
- Uncommon (may affect up to 1 in 100 people):
- depression,
- mood changes,
- sleep disorders,
- dry mouth,
- intense itching or severe skin rashes),
- blistering of the skin,
- kidney problems,
- impotence,
- sweating,
- excess eosinophils (a type of white blood cell),
- drowsiness,
- fainting,
- palpitations,
- tachycardia,
- vasculitis (inflammation of blood vessels),
- photosensitivity reactions (increased skin sensitivity to sunlight),
- arthralgia (joint pain),
- myalgia (muscle pain),
- chest pain,
- general malaise,
- peripheral edema,
- fever,
- fall,
- change in analytical values: high potassium levels in blood reversible by stopping treatment, low sodium levels, hypoglycemia in diabetic patients (very low blood sugar levels), elevated blood urea, and elevated creatinine in blood.
- Rare (may affect up to 1 in 1,000 people):
- acute kidney failure,
- concentrated urine, feeling of discomfort (nausea) or being sick (vomiting), muscle cramps, confusion, and convulsions. These symptoms may be due to a disease called SIADH (inadequate secretion of antidiuretic hormone),
- decreased or absent diuresis
- facial flushing,
- worsening of psoriasis,
- changes in laboratory parameters: increased liver enzymes, high bilirubin levels.
- Very Rare (may affect up to 1 in 10,000 people):
- confusion,
- eosinophilic pneumonia (a rare type of pneumonia),
- rhinitis (nasal congestion or runny nose),
- changes in blood parameters such as decreased white and red blood cell count, decreased hemoglobin concentration, decreased platelet count.
If you have these symptoms, contact your doctor as soon as possible.
Frequency not known (cannot be estimated from available data): Change in color, numbness, and pain in the fingers of the hands or feet (Raynaud's disease).
Reporting of Adverse Effects
If you experience any type of adverse effect, consult your doctor, even if it is a possible adverse effect that does not appear in this prospectus. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of Coversoral
Keep this medicine out of sight and reach of children.
Do not use this medicine after the expiration date that appears on the box and packaging. The expiration date is the last day of the month indicated.
Keep the packaging perfectly closed to protect it from moisture.
Medicines should not be thrown away through drains or into the trash. Deposit the packaging and medicines you no longer need at the SIGRE Point of your usual pharmacy. In case of doubt, ask your pharmacist how to dispose of the packaging and medicines you no longer need. This way, you will help protect the environment.
6. Package Contents and Additional Information
Composition of Coversoral 5 mg
- The active ingredient is perindopril arginine. Each orodispersible tablet contains 3.395 mg of perindopril (which corresponds to 5 mg of perindopril arginine).
The other components of the orodispersible tablet are: magnesium stearate (E470B), anhydrous colloidal silica (E551), dry powder composed of lactose and starch (lactose monohydrate 85%, cornstarch 15%), aspartame (E951), and potassium acesulfame (E950).
- The appearance of the product and packaging content:
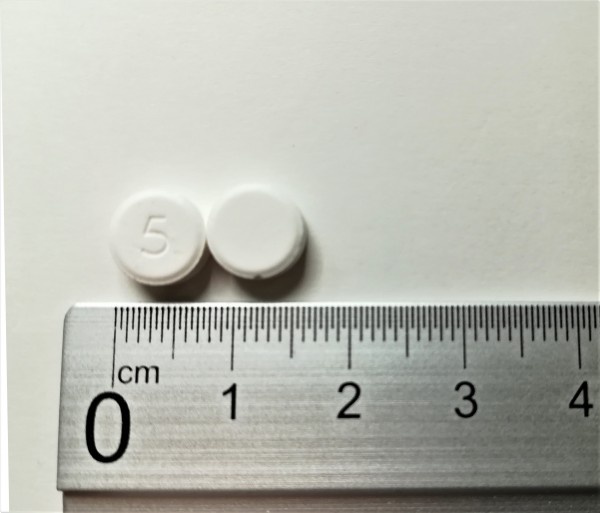
The Coversoral 5 mg orodispersible tablets are white and round.
The tablets are available in packages of 5, 10, 14, 20, 30, 50, 60, 90, 100, 120, or 500 tablets.
Only some package sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder
Les Laboratoires Servier
50, rue Carnot
92284 Suresnes cedex - France
Manufacturer
Les Laboratoires Servier Industrie
905 route de Saran
45520 Gidy - France
and
Servier (Ireland) Industries Ltd
Gorey Road
Arklow - Co. Wicklow - Ireland
and
ANPHARM Przedsiebiorstwo Farmaceutyczne S.A.
UI. Annopol 6B-03-236
Warsaw - Poland
This medicine is authorized in the Member States of the European Economic Area under the following names:
Bulgaria | PRESTARIUM 5 mg ????????, ???????????? ?? ? ?????? |
Estonia | Prestarium Arginine 5 mg suus dispergeeruv tablett |
Slovakia | PRESTARIUM A 5 mg orodispergovatelná tableta |
Slovenia | BIOPREXANIL 5 mg orodisperzibilne tablete |
Spain | COVERSORAL 5 mg |
France | COVERSYL 5 mg comprimé orodispersible |
Ireland | COVERSYL Arginine 5 mg Orodispersible tablets |
Latvia | PRESTARIUM 5 mg mute dispergejamas tabletes |
Lithuania | PRESTARIUM 5 mg burnoje disperguojamosios tabletes |
Portugal | COVERSORAL 5 mg comprimidos orodispersíveis |
Czech Republic | PRESTARIUM NEO ORODISPERZNÍ tablety |
Date of the last revision of this prospectus: October 2021
Detailed information about this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to COVERSORAL 5 mg ORALLY DISPERSIBLE TABLETSDosage form: ORALLY DISINTEGRATING TABLET/LYOTAB, 10 mgActive substance: perindoprilManufacturer: Les Laboratoires ServierPrescription requiredDosage form: TABLET, 4 mgActive substance: perindoprilManufacturer: Aurovitas Spain, S.A.U.Prescription requiredDosage form: TABLET, 4 MGActive substance: perindoprilManufacturer: Krka D.D. Novo MestoPrescription required
Online doctors for COVERSORAL 5 mg ORALLY DISPERSIBLE TABLETS
Discuss questions about COVERSORAL 5 mg ORALLY DISPERSIBLE TABLETS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions











