
SODIUM CHLORIDE MEINSOL 9 mg/ml SOLUTION FOR PARENTERAL USE

How to use SODIUM CHLORIDE MEINSOL 9 mg/ml SOLUTION FOR PARENTERAL USE
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Prospective: Information for the User
Sodium Chloride Meinsol 9 mg/ml Solvent for Parenteral Use
Sodium Chloride
Read the entire prospectus carefully before starting to use this medication, as it contains important information for you.
- Keep this prospectus, as you may need to read it again.
- If you have any doubts, consult your doctor or pharmacist.
- This medication has been prescribed to you only, and you should not give it to others,
even if they have the same symptoms, as it may harm them.
- If you experience adverse effects, consult your doctor or pharmacist, even if they are not listed in this prospectus. See section 4.
Contents of the Prospectus:
- What Sodium Chloride Meinsolis and what it is used for
- What you need to know before starting to use Sodium Chloride Meinsol
- How to use Sodium Chloride Meinsol
- Possible adverse effects
- Storage of Sodium Chloride Meinsol
- Package contents and additional information.
1. What is Sodium Chloride Meinsol and what is it used for
Sodium Chloride Meinsol is indicated as a solvent for medications that must be administered
intravenously, intramuscularly, or subcutaneously, and as a support for the addition of medications.
2. What you need to know before starting to use Sodium Chloride Meinsol
Do not use Sodium Chloride Meinsol:
- If you have had any allergic or unusual reaction to sodium chloride.
- If you have a high concentration of sodium in the blood (hypernatremia)
- If you have increased muscle tone (hypertonia)
- If you suffer from heart failure (inability of the heart to pump the necessary amount of blood)
- If you have any heart, liver, or kidney disorder and suffer from water accumulation (edema) in the body
- If you have severe high blood pressure (severe hypertension)
- If you have an excess of acid in the blood (metabolic acidosis)
Warnings and Precautions
Consult your doctor or pharmacist before starting to use Sodium Chloride Meinsol
- The solutions, once opened, must be used immediately.
- In the case of subcutaneous administration, do not add any supplement that may change the isotonicity of the solution.
- Do not use the solution if it is not transparent and free of precipitates.
- Ensure the physical-chemical compatibility when adding medication to the ampoule.
- The addition of alcohol to sodium chloride solutions should be avoided.
Children
Newborns may present excessively high levels of sodium due to the immaturity of their kidneys. Therefore, repeated injections of sodium chloride can only be administered to them once the sodium levels in the blood have been determined.
Sodium chloride should be used with caution in patients with hypertension, heart failure, pulmonary or peripheral edema, altered renal function, pre-eclampsia, hyperaldosteronism, cirrhosis, and other liver diseases, hypervolemia, urinary tract obstruction, hypoproteinemia, and other diseases and treatments (e.g., corticosteroids) associated with sodium retention.
Use of Sodium Chloride Meinsol with other medications:
Inform your doctor or pharmacist if you are taking, have recently taken, or may need to take any other medication.
Interactions with other medications depend on the medication added.
The 9 mg/ml sodium chloride solution presents incompatibilities with hydrocortisone, amphotericin B, tetracyclines, cephalotin, erythromycin, lactobionate, and lithium salts.
It is incompatible with active principles that are not soluble in the sodium chloride solution, due to possible precipitation of the active principle, as well as with medications whose stability or solubility require a very acidic or strongly alkaline pH.
Pregnancy and Breastfeeding:
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using a medication.
Given the characteristics of the product, no effect on pregnant or breastfeeding women is expected, provided that administration is correct and controlled.
Driving and Using Machines:
There is no indication that the isotonic sodium chloride solution may affect the ability to drive or use machines.
3. How to use Sodium Chloride Meinsol
Follow these instructions unless your doctor has given you different instructions. Sodium Chloride Meinsol is administered intravenously, intramuscularly, or subcutaneously.
It is not necessary to sterilize the ampoule before opening it.
No cutting element is needed to open the ampoule.
Once the ampoule is opened, the nozzle fits perfectly onto the cone of the syringe (Luer cone), so it is not necessary to use a needle.
To open:
To separate an ampoule from the rest, turn one ampoule on itself against the rest of the ampoules in the strip without touching the head and neck of the ampoules (1). Shake the ampoule with a single movement as shown in the drawing to eliminate any liquid that may be in the stopper (2). To open the ampoule, turn the body and head of the ampoule in opposite directions until it breaks at the neck (3). Connect the Luer or Luer-lock syringe as shown in the drawing (4).
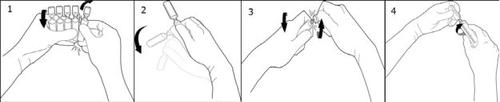
So, no needle is needed to extract the solution. Extract the liquid.
The solution does not contain any type of preservative or bactericide, so opened and unused ampoules must be discarded immediately.
The amount to be used will vary depending on the concentration to be administered of the medication to be dissolved.
Your doctor will indicate the duration of treatment with your medication.
If you think the effect of Sodium Chloride Meinsol is too strong or weak, inform your doctor.
If you use more Sodium Chloride Meinsol than you need:
If you have received more Sodium Chloride Meinsol than you need, consult your doctor immediately.
Given the nature of the product, if its indication and administration are correct and controlled, there is no risk of intoxication.
However, an excess of sodium chloride, in its most acute form, produces dehydration of the internal organs, nausea, vomiting, diarrhea, abdominal cramps, thirst, decreased salivation and tearing, sweating, fever, hypotension, tachycardia, renal failure, pulmonary edema, acidosis, respiratory arrest, headache, dizziness, irritability, muscle spasms, stiffness, coma, and death.
In case of symptoms of intoxication, administration will be suspended, and symptomatic treatment will be resorted to.
In children, coma and convulsions can persist until vascular lesions are produced. Difficulty breathing with tachypnea and redness of the nostrils may also appear.
In case of overdose or accidental ingestion, go immediately to a medical center or call the Toxicology Information Service at 91 562 04 20.
4. Possible Adverse Effects
Like all medications, this medication can produce adverse effects, although not all people experience them.
The inadequate or excessive administration of physiological saline solution can produce hyperhydration, hypernatremia, hyperchloremia, and related manifestations, such as metabolic acidosis, due to the decrease in bicarbonate concentration, and edema formation.
An excess of sodium chloride can produce nausea, vomiting, headache.
Adverse reactions can also be related to the added medication.
Reporting Adverse Effects
If you experience any type of adverse effect, consult your doctor or pharmacist, even if it is a possible adverse effect that is not listed in this prospectus. You can also report them directly through the Spanish Medication Surveillance System for Human Use, http://www.notificaram.es. By reporting adverse effects, you can contribute to providing more information on the safety of this medication.
5. Storage of Sodium Chloride Meinsol
Keep this medication out of the sight and reach of children.
No special storage conditions are required.
Do not use this medication after the expiration date shown on the packaging after CAD. The expiration date is the last day of the month indicated.
Once the package is opened, the product must be used immediately.
6. Package Contents and Additional Information
- The active ingredient is Sodium Chloride. Each 100 ml contains 0.9 g of sodium chloride.
- The excipients are water for injectable preparations, hydrochloric acid, and sodium hydroxide.
Centesimal composition:
Electrolytes mmol/l mEq/l
Sodium Chloride 0.9 g Na+ 154 154
Water for injectable preparations c.s.p. 100 ml Cl- 154 154
Osmolarity: 308 mOsmol/liter.
Appearance of the Product and Package Contents
Sodium Chloride Meinsol 9 mg/ml solvent for parenteral use is a clear and colorless solution, free or practically free of particles.
Sodium Chloride Meinsol is a solvent for parenteral use that is presented in the following formats:
Box with 20 ampoules of 5 ml (Clinical Package) Box with 50 ampoules of 5 ml (Clinical Package) Box with 20 ampoules of 10 ml (Clinical Package) Box with 50 ampoules of 10 ml (Clinical Package) Box with 20 ampoules of 20 ml (Clinical Package)
Not all package sizes may be marketed.
Marketing Authorization Holder:
FRESENIUS KABI ESPAÑA, S.A.U.
C/ Marina, 16-18
08005-Barcelona (Spain)
Manufacturer:
FRESENIUS KABI ESPAÑA, S.A.U.
C/ Dr. Ferran, 12
08339 Vilassar de Dalt
Spain
This medication is authorized in the member states of the European Economic Area with the following names:
Member State Name | Medication Name |
Belgium | Natriumchloride 0,9% Fresenius Kabi solvent for parenteral use |
Czech Republic | 0,9% Sodium Chloride Kabi |
Estonia | Sodium chloride Kabi 0,9%, solvent for parenteral use |
Greece | Sodium Chloride 0.9%/Fresenius |
Hungary | Nátrium-klorid Kabi 9mg/ml oldószer parenterális készítményekhez |
Ireland | Sodium Chloride 0.9% w/v solvent for parenteral use |
Lithuania | Sodium Chloride Kabi 0,9 % tirpiklis parenteriniam vartojimui |
Latvia | Sodium chloride Kabi 0,9% šķīdinātājs parenterālai lietošanai |
Poland | Natrium chloratum 0,9% Kabi, 9 mg/ml, rozpuszczalnik do sporządzania leków parenteralnych |
Romania | Ser fiziologic 9 mg/ml Kabi solvent pentru uz parenteral |
Slovak Republic | 0,9 % Sodium chloride Kabi |
Slovenia | Natrijev klorid Fresenius Kabi 9 mg/ml |
Spain | Cloruro de sodio Meinsol 9 mg/ml disolvente para uso parenteral |
Date of the last revision of this prospectus: January 2015
Detailed and updated information about this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to SODIUM CHLORIDE MEINSOL 9 mg/ml SOLUTION FOR PARENTERAL USEDosage form: INJECTABLE, 900 mg sodium chloride / 100 mlActive substance: Solvents and diluting agents, incl. irrigating solutionsManufacturer: B Braun Medical S.A.Prescription requiredDosage form: INJECTABLE, 1 mlActive substance: Solvents and diluting agents, incl. irrigating solutionsManufacturer: B Braun Medical S.A.Prescription requiredDosage form: INJECTABLE, q.s. 5 mlActive substance: Solvents and diluting agents, incl. irrigating solutionsManufacturer: Oiarso Sociedad CooperativaPrescription required
Online doctors for SODIUM CHLORIDE MEINSOL 9 mg/ml SOLUTION FOR PARENTERAL USE
Discuss questions about SODIUM CHLORIDE MEINSOL 9 mg/ml SOLUTION FOR PARENTERAL USE, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions







