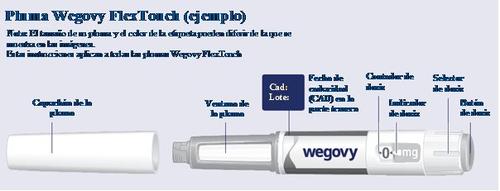
WEGOVY 0.5 mg FLEXTOUCH PRE-FILLED PEN INJECTABLE SOLUTION

How to use WEGOVY 0.5 mg FLEXTOUCH PRE-FILLED PEN INJECTABLE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Wegovy 0.25mg FlexTouch solution for injection in pre-filled pen
Wegovy 0.5mg FlexTouch solution for injection in pre-filled pen
Wegovy 1mg FlexTouch solution for injection in pre-filled pen
Wegovy 1.7mg FlexTouch solution for injection in pre-filled pen
Wegovy 2.4mg FlexTouch solution for injection in pre-filled pen
semaglutide
This medicine is subject to additional monitoring, which will allow for quick identification of new safety information. You can help by reporting any side effects you may get. The last section of this leaflet includes information on how to report side effects.
Read all of this leaflet carefully before you start using this medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Wegovy and what is it used for
- What you need to know before you use Wegovy
- How to use Wegovy
- Possible side effects
- Storage of Wegovy
- Contents of the pack and other information
1. What is Wegovy and what is it used for
What is Wegovy
Wegovy is a medicine for weight loss and weight maintenance that contains the active substance semaglutide. It is similar to a naturally occurring hormone called glucagon-like peptide-1 (GLP-1) that is released from the intestine after eating. It works by acting on areas (receptors) of the brain that control appetite, making you feel fuller and less hungry, and experiencing less desire to eat. This will help you eat fewer calories and reduce body weight. Wegovy may also help prevent heart disease.
What Wegovy is used for
Wegovy is used together with diet and physical activity to lose weight and help keep weight under control. It is used in adults who have
- a BMI of 30 kg/m² or higher (obesity) or
- a BMI of at least 27 kg/m², but less than 30 kg/m² (overweight) who have health problems related to weight (such as diabetes, high blood pressure, abnormal blood fats, breathing problems during sleep called "obstructive sleep apnea" or a history of heart attack, stroke, or vascular problems).
Body Mass Index (BMI) is a measure of weight in relation to height.
Wegovy is used together with diet and physical activity for weight control in adolescents aged 12 years and older with
- obesity and
- body weight >60 kg.
As an adolescent patient, you should only continue using Wegovy if you have lost at least 5% of your BMI after 12 weeks of treatment with the 2.4 mg dose or the maximum tolerated dose (see section 3). Consult your doctor before continuing.
2. What you need to know before you use Wegovy
Do not use Wegovy
- if you are allergic to semaglutide or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before you start using Wegovy.
Wegovy should not be used if:
- you use other products for weight loss,
- you have type 1 diabetes,
- you have severely reduced kidney function,
- you have severely reduced liver function,
- you have severe heart failure,
- you have diabetic eye disease (retinopathy).
There is limited experience with Wegovy in patients:
- 85 years of age or older,
- with liver problems,
- with severe stomach or intestinal problems that cause delayed emptying of the stomach (called gastroparesis) or if you have any inflammatory bowel disease.
Talk to your doctor if you are in any of these situations.
If you know you are going to have surgery where you will be under anesthesia (sleep), tell your doctor that you are taking Wegovy.
- Dehydration
During treatment with Wegovy, you may have nausea, vomiting, or diarrhea. These side effects can cause dehydration (loss of fluids). It is important to drink enough fluids to prevent dehydration. This is especially important if you have kidney problems. If you have any doubts or concerns, talk to your doctor.
- Pancreatitis
If you have severe and persistent stomach pain (see section 4), go to the doctor immediately, as it could be a symptom of pancreatitis (acute pancreatitis).
- People with type 2 diabetes
Wegovy cannot be used as a substitute for insulin. Do not use Wegovy in combination with other medicines that contain GLP-1 receptor agonists (such as liraglutide, dulaglutide, exenatide, or lixisenatide).
- Low blood sugar (hypoglycemia)
Taking a sulfonylurea or insulin with Wegovy may increase the risk of experiencing low blood sugar (hypoglycemia). To know the warning signs of low blood sugar, see section 4. Your doctor may ask you to measure your blood sugar levels. This will help your doctor decide if it is necessary to change the dose of sulfonylurea or insulin to reduce the risk of low blood sugar.
- Diabetic eye disease (retinopathy)
If you have diabetic eye disease and are using insulin, this medicine may cause your vision to worsen and you may need treatment. Sudden improvements in blood sugar control can cause a temporary worsening of diabetic eye disease. Tell your doctor if you have diabetic eye disease and experience eye problems while taking this medicine.
Children and adolescents
The safety and efficacy of Wegovy in children under 12 years of age have not been studied, and its use is not recommended in this population.
Other medicines and Wegovy
Tell your doctor, pharmacist, or nurse if you are using, have recently used, or might use any other medicines.
In particular, tell your doctor, pharmacist, or nurse if you are using medicines that contain:
- warfarin or other similar medicines taken by mouth to reduce blood clotting (oral anticoagulants). When you start treatment, for example, with warfarin or similar medicines, you may need to have frequent blood tests to determine your blood clotting ability.
Pregnancy and breastfeeding
This medicine should not be used during pregnancy, as it is not known if it can affect the fetus. Therefore, it is recommended to use contraceptive methods during the use of this medicine. If you wish to become pregnant, you should stop using this medicine at least 2 months in advance. If you become pregnant, think you may be pregnant, or plan to become pregnant while using this medicine, talk to your doctor immediately, as you should stop treatment.
Do not use this medicine during breastfeeding, as it is not known if it is excreted in breast milk.
Driving and using machines
Wegovy is unlikely to affect your ability to drive or use machines. Some patients may feel dizzy when using Wegovy, mainly during the first 4 months of treatment (see section 4). If you feel dizzy, be extra careful when driving or using machines. If you need more information, talk to your doctor, pharmacist, or nurse.
People with type 2 diabetes
If you use this medicine in combination with a sulfonylurea or insulin, you may experience low blood sugar (hypoglycemia), which can reduce your ability to concentrate. Avoid driving or using machines if you experience any symptoms of low blood sugar. See section 2, "Warnings and precautions" for information on the increased risk of low blood sugar and section 4 to know the warning signs of low blood sugar. For more information, talk to your doctor.
Wegovy contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per dose; it is essentially "sodium-free".
3. How to use Wegovy
Follow the instructions for administration given by your doctor exactly. If you are in doubt, talk to your doctor, pharmacist, or nurse again.
How much to use
Adults
The recommended dose is 2.4 mg once a week.
Treatment will start with a low dose that will be gradually increased over 16 weeks of treatment.
- When you start using Wegovy, the starting dose is 0.25 mg once a week.
- Your doctor will tell you to gradually increase the dose every 4 weeks until you reach the recommended dose of 2.4 mg once a week.
- Once you reach the recommended dose of 2.4 mg, do not increase the dose further.
- If you feel very bothered by nausea or vomiting, talk to your doctor about the possibility of delaying the dose escalation or going back to the previous dose until the symptoms have improved.
Normally, you will be told to follow the following table.
Dose escalation | Weekly dose |
Week 1 to 4 | 0.25 mg |
Week 5 to 8 | 0.5 mg |
Week 9 to 12 | 1 mg |
Week 13 to 16 | 1.7 mg |
From week 17 | 2.4 mg |
Your doctor will regularly assess your treatment.
Adolescents (12 years and older)
For adolescents, the same dose escalation schedule should be applied as for adults (see above). The dose should be increased to 2.4 mg (maintenance dose) or to the maximum tolerated dose. Weekly doses above 2.4 mg are not recommended.
How to administer Wegovy
Wegovy is administered as an injection under the skin (subcutaneous injection). Do not inject it into a vein or muscle.
- The best areas for injection are the front of the upper arm, the top of the thighs, or the stomach.
- Before using the pen for the first time, your doctor, pharmacist, or nurse will show you how to use it.
On the other side of this leaflet, you will find detailed instructions on how to use the pen.
People with type 2 diabetes
Tell your doctor if you have type 2 diabetes. Your doctor may adjust the dose of your diabetes medication to avoid low blood sugar.
When to use Wegovy
- You should use this medicine once a week, and if possible, on the same day of the week.
- The injection can be given at any time of day, regardless of meals.
If necessary, you can change the day of the weekly injection of this medicine, as long as at least 3 days have passed since the last injection. Once you have selected a new injection day, you should continue with the weekly dosing schedule.
If you use more Wegovy than you should
Talk to your doctor immediately. You may experience side effects such as nausea, vomiting, or diarrhea, which can cause dehydration (loss of fluids).
If you forget to use Wegovy
If you forget to inject a dose and:
- it has been 5 days or less since you should have used Wegovy, use it as soon as you remember. Then, inject the next dose as usual, on the scheduled day.
- it has been more than 5 days since you should have used Wegovy, skip the missed dose. Then, inject the next dose as usual, on the next scheduled day.
Do not give a double dose to make up for forgotten doses.
If you stop using Wegovy
Do not stop using this medicine without talking to your doctor.
If you have any other questions about the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Serious side effects
Common(may affect up to 1 in 10 people)
- Complications of diabetic eye disease (diabetic retinopathy). If you have diabetes, you should tell your doctor if you experience eye problems, such as changes in vision, during treatment with this medicine.
Uncommon(may affect up to 1 in 100 people)
- Pancreatitis (acute pancreatitis). Symptoms of pancreatitis can include severe and persistent stomach pain, which can radiate to the back. If you experience such symptoms, go to the doctor immediately.
Rare(may affect up to 1 in 1,000 people)
- Severe allergic reactions (anaphylactic reactions, angioedema). You should seek medical help and tell your doctor immediately if you experience symptoms such as difficulty breathing, swelling, dizziness, rapid heartbeat, sweating, and loss of consciousness or rapid swelling under the skin in areas such as the face, throat, arms, and legs, which can be potentially fatal if the swelling of the throat blocks the airways.
Frequency not known(cannot be estimated from the available data)
- Intestinal obstruction. A severe form of constipation with other symptoms such as stomach pain, abdominal swelling, vomiting, etc.
Other side effects
Very common(may affect more than 1 in 10 people)
- headache
- nausea
- vomiting
- diarrhea
- constipation
- stomach pain
- feeling weak or tired
- these are mainly seen during dose escalation and usually disappear over time.
Common(may affect up to 1 in 10 people)
- feeling dizzy
- stomach upset or indigestion
- belching
- gas (flatulence)
- stomach swelling
- inflamed stomach (gastritis); symptoms include stomach pain, nausea, or vomiting
- reflux or heartburn; also called "gastroesophageal reflux disease"
- gallstones
- hair loss
- reactions at the injection site
- change in taste of food or drink
- change in skin sensation
- low blood sugar (hypoglycemia) in patients with type 2 diabetes.
The warning signs of low blood sugar may appear suddenly. They can include cold sweat, pale and cold skin, headache, rapid heartbeat, nausea, or excessive hunger, changes in vision, drowsiness, or feeling weak, nervousness, anxiety, or confusion, difficulty concentrating, or tremors.
Your doctor will tell you how to treat low blood sugar and what to do if you notice these warning signs.
It is more likely that your blood sugar will be low if you are also using a sulfonylurea or insulin. Your doctor may reduce the dose of these medicines before you start using this medicine.
Uncommon(may affect up to 1 in 100 people)
- low blood pressure
- feeling dizzy or lightheaded when standing up or sitting down due to a drop in blood pressure
- rapid heartbeat
- increased pancreatic enzymes (such as lipase and amylase) in blood tests
- a delay in emptying the stomach.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Wegovy
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label of the pen and on the carton after "EXP". The expiry date is the last day of the month stated.
Before opening
Store in a refrigerator (between 2°C and 8°C). Do not freeze. Keep away from the cooling element of the refrigerator.
During use
- The pen can be stored for 6 weeks if stored at a temperature not above 30°C or in a refrigerator (between 2°C and 8°C) away from the cooling element. Do not freeze Wegovy and do not use if it has been frozen.
- When not in use, keep the pen cap on to protect it from light.
Do not use this medicine if you notice that the solution is not clear and colorless.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
Composition of Wegovy
- The active ingredient is semaglutide.
Wegovy 0.25 mg FlexTouch injectable solution
Each pre-filled pen contains 1 mg of semaglutide in 1.5 ml (0.68 mg/ml).
Wegovy 0.5 mg FlexTouch injectable solution
1.5 ml: each pre-filled pen contains 2 mg of semaglutide in 1.5 ml (1.34 mg/ml).
3 ml: each pre-filled pen contains 2 mg of semaglutide in 3 ml (0.68 mg/ml).
Wegovy 1 mg FlexTouch injectable solution
Each pre-filled pen contains 4 mg of semaglutide in 3 ml (1.34 mg/ml).
Wegovy 1.7 mg FlexTouch injectable solution
Each pre-filled pen contains 6.8 mg of semaglutide in 3 ml (2.27 mg/ml).
Wegovy 2.4 mg FlexTouch injectable solution
Each pre-filled pen contains 9.6 mg of semaglutide in 3 ml (3.2 mg/ml).
- The other components are disodium phosphate dihydrate, propylene glycol, phenol, hydrochloric acid/sodium hydroxide (for pH adjustment), and water for injectable preparations. See also section 2 "Wegovy contains sodium" for more information on sodium.
Appearance of Wegovy and Container Contents
Wegovy is a clear and colorless injectable solution in a pre-filled pen.
Each pre-filled pen contains 4 doses.
Wegovy 0.25, 0.5, 1, and 1.7 mg FlexTouch injectable solution is available in the following package size:
1 pre-filled pen and 4 NovoFine Plus disposable needles.
Wegovy 2.4 mg FlexTouch injectable solution is available in the following package sizes:
1 pre-filled pen and 4 NovoFine Plus disposable needles.
3 pre-filled pens and 12 NovoFine Plus disposable needles
Not all package sizes may be marketed.
Marketing Authorization Holder
Novo Nordisk A/S
Novo Allé
DK-2880 Bagsværd
Denmark
Manufacturer
Novo Nordisk A/S
Novo Allé
DK-2880 Bagsværd
Denmark
Novo Nordisk Production SAS
45, Avenue d’Orléans
28000 Chartres
France
Date of Last Revision of this Leaflet:
Other Sources of Information
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu.
Instructions for Using the Wegovy Pen | |
Read these Instructions Carefullybefore starting to use your Wegovy FlexTouch pen weekly and consult your doctor, nurse, or pharmacist on how to inject Wegovy correctly. The Wegovy pen is a dosing pen that contains your four prescribed doses of Wegovy, corresponding to the four weekly administrations. Use the table found inside the outer packaging cover to keep track of how many injections you have used and how many doses are left in the pen. Wegovy comes in five different pens, each containing one of the following prescribed doses of semaglutide:
Always Check the Pen Labelto ensure it contains the prescribed dose of Wegovy. Your pen is designed to be used with 30G, 31G, and 32G disposable needles up to 8 mm in length. The Package Contains:
| |
| |
| |
1 Prepare the Pen with a New Needle | |
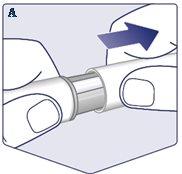
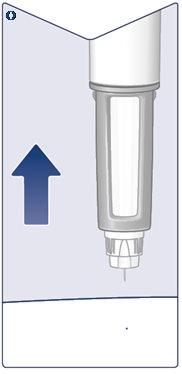
Check the Name and Concentrationof your pen to ensure it contains the prescribed dose of Wegovy. Remove the Pen Cap. (See Figure A). |
|
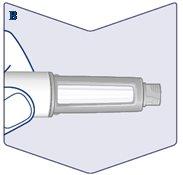
Check that the Solution in Your Pen is Clear and Colorless. Look through the pen window. If Wegovy appears cloudy or discolored, do not use the pen. (See Figure B). |
|
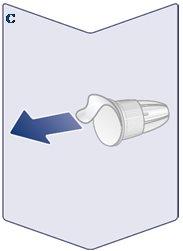
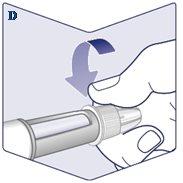
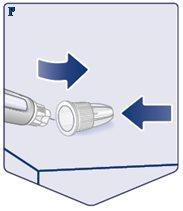
Always Use a New Needle for Each Injection. Take a Needlewhen you are ready to give the injection. Check that the paper tab and the outer cap of the needle are not damaged, which could affect sterility. If you find any damage, use a new needle. Remove the Paper Tab. (See Figure C). |
|
Place the Needle Straight onto the Pen. Screw it on until it is Secure. (See Figure D). |
|
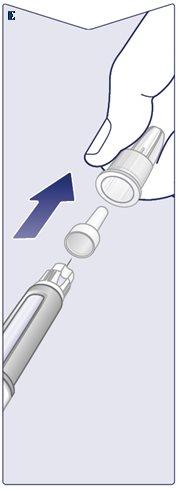
The Needle is Covered by Two Caps. You Must Remove Both Caps. If you forget to remove both caps, you will not inject Wegovy. Remove the Outer Needle Cap and Set it Aside.You will need it later to safely remove the needle from the pen. Remove the Inner Needle Cap and Discard it.A drop of Wegovy may appear at the tip of the needle. You should continue to check the flow of Wegovy if you are using a new pen for the first time. See “Check the Flow with Each New Pen”. Never use a bent or damaged needle. For more information on handling needles, see the section “About Needles”that follows these instructions. (See Figure E). |
|
Check the Flow with Each New Pen | |
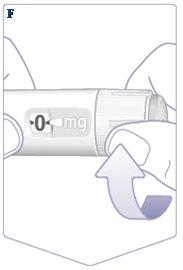
If your Wegovy pen is already in use, go to “2 Selecting the Dose”. Check the flow of Wegovy only before the first injection with each new pen. Turn the dose selector until you see the flow check symbol () (See Figure F). |
|
Make sure the flow check symbol matches the dose indicator. (See Figure G). |
|
Check the Flow | |
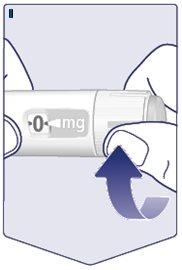
Hold the pen with the needle pointing upwards. Press and Hold the Dose Buttonuntil the dose counter returns to . The must match the dose indicator. A drop of Wegovy should appear at the tip of the needle. This drop indicates that the pen is ready for use. If a drop does not appear, check the flow again. This should only be done twice. If a drop still does not appear, change the needle and check the flow again. Do Not Use the Penif a drop of Wegovy still does not appear. (See Figure H). |
|
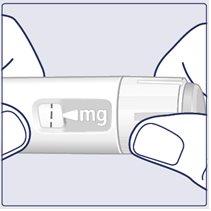
2 Selecting the Dose | |
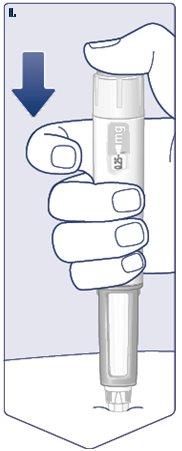
Turn the dose selector until the dose counter stopsand shows the selected dose. (See Figure I). |
|
The dashed line () on the dose counter will guide you to your dose. The dose selector clicks differently when turned forward, backward, or when passing the dose. You will hear a “click” each time you turn the dose selector. Do not count the pen clicks. (See Figure J). |
|
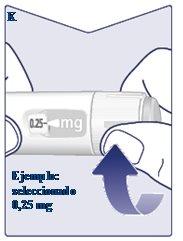
When the Selected Dose Matches the Dose Indicator, You Have Selected the Dose.In this image, the dose is shown as an example. If the dose counter stops before reaching the prescribed dose, see the section “Do You Have Enough Wegovy?”that follows these instructions. (See Figure K). |
|
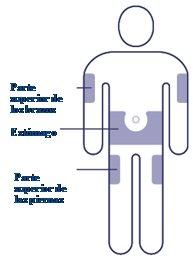
Choose the Injection Site Choose the upper arm, upper thigh, or stomach (keeping a distance of 5 cm from the navel). You can inject in the same body area each week, but make sure it is not in the same spot you used last time. |
|
3 Injecting the Dose | |
Insert the Needle Under the Skin. Make Sure You Can See the Dose Counter.Do not touch the dose counter with your fingers. This could interrupt the injection. (See Figure L). |
|
Press and Hold the Dose Buttonuntil appears on the dose counter. (See Figure M). Press and Hold the Dose Buttonwith the needle under the skin and count slowly to 6. The must match the dose indicator. You may hear or feel a click when the dose counter returns to . (See Figure N). |
|
Remove the Needle from the Skin.If the needle is removed too early, a jet of Wegovy may come out of the needle tip, and the full dose may not be administered. If blood appears at the injection site, press the area lightly to stop the bleeding. You may see a drop of Wegovy on the needle tip after the injection. This is normal and does not affect the dose. (See Figure O). |
|
4 After the Injection | |
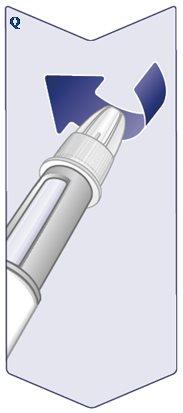
Insert the Needle Tip into the Outer Needle Capon a flat surface without touching the needle or the outer needle cap. When the Needle is Covered,carefully press the outer needle cap fully. (See Figure P). |
|
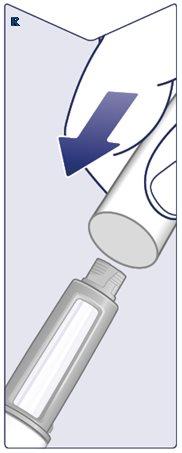
Unscrew the Needleand carefully dispose of it according to the doctor's, nurse's, pharmacist's, or local authorities' instructions. Never Attempt to Put the Inner Needle Cap Back On,as you could prick yourself with it. Always Dispose of the Needle Immediately After Each Injectionto avoid needle obstruction, contamination, infection, and incorrect dosing. Never Store the Pen with the Needle Attached. (See Figure Q). |
|
Put the Pen Cap Back Onafter each use to protect Wegovy from light. (See Figure R). |
|
When the pen is empty, dispose of it without a needle, following the doctor's, nurse's, pharmacist's, or local authorities' instructions. The pen cap and empty box can be discarded in household trash. | |
About Needles | |
How to Identify a Blocked or Damaged Needle
How to Proceed with a Blocked Needle
| |
Maintenance of the Pen | |
Treat your pen with care. Rough handling or misuse can cause inaccurate dosing. If this happens, you may not get the desired effect of Wegovy.
| |
Do You Have Enough Wegovy? | |
If the dose counter stops before reaching the prescribed dose, there is not enough Wegovy for a full dose. Discard the pen and use a new Wegovy pen. |
|
Important Information | |
|
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to WEGOVY 0.5 mg FLEXTOUCH PRE-FILLED PEN INJECTABLE SOLUTIONDosage form: INJECTABLE, 0.25 mgActive substance: semaglutideManufacturer: Novo Nordisk A/SPrescription requiredDosage form: INJECTABLE, 0.5 mgActive substance: semaglutideManufacturer: Novo Nordisk A/SPrescription requiredDosage form: INJECTABLE, 1 mgActive substance: semaglutideManufacturer: Novo Nordisk A/SPrescription required
Online doctors for WEGOVY 0.5 mg FLEXTOUCH PRE-FILLED PEN INJECTABLE SOLUTION
Discuss questions about WEGOVY 0.5 mg FLEXTOUCH PRE-FILLED PEN INJECTABLE SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions