VOXZOGO 0.4 mg POWDER AND SOLVENT FOR INJECTABLE SOLUTION

How to use VOXZOGO 0.4 mg POWDER AND SOLVENT FOR INJECTABLE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Voxzogo 0.4 mg powder and solvent for solution for injection
Voxzogo 0.56 mg powder and solvent for solution for injection
Voxzogo 1.2 mg powder and solvent for solution for injection
vosoritide
This medicine is subject to additional monitoring, which will allow for the quick identification of new safety information. You can help by reporting any side effects you may have experienced or that your child may have experienced. The last section of section 4 will provide information on how to report side effects.
Read all of this leaflet carefully before you or your child start using this medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you or your child have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed to you or your child only. Do not give it to others, as it may harm them, even if their symptoms are the same as yours.
- If you or your child experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the package leaflet
- What is Voxzogo and what is it used for
- What you need to know before you or your child use Voxzogo
- How to use Voxzogo
- Possible side effects
- Storage of Voxzogo
- Contents of the pack and other information
1. What is Voxzogo and what is it used for
What is Voxzogo
Voxzogo contains the active substance vosoritide. It is similar to a protein found in the body called C-type natriuretic peptide (CNP). Vosoritide is produced using recombinant technology, where modified bacteria are used to include the gene that produces the protein.
What Voxzogo is used for
This medicine is used to treat achondroplasia in patients aged 4 months and older whose bones are still growing. Achondroplasia is a genetic disorder that affects the growth of almost all bones in the body, including the bones of the skull, spine, arms, and legs, resulting in a significantly reduced height with a characteristic appearance.
This product is only indicated for achondroplasia caused by a mutation in the FGFR3 gene, confirmed by genetic testing.
How Voxzogo works
The active substance in Voxzogo works directly on the growth plates of the bones to promote the growth of new bone.
2. What you need to know before you or your child use Voxzogo
Do not use Voxzogo
- if you or your child are allergic to vosoritide or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor before starting Voxzogo:
- if you or your child have significant heart or blood pressure problems;
- if you or your child are taking or have recently taken medicines to lower blood pressure.
If any of the above applies to you or your child, or if you are unsure, talk to your doctor before using Voxzogo.
Effects on blood pressure
Voxzogo may lower blood pressure. As a result, you may feel dizzy, tired, or nauseous. Usually, blood pressure returns to normal within 90 minutes after the Voxzogo injection. If these effects occur and are severe, tell your doctor.
Drinking plenty of fluids at the time of injection may reduce the likelihood of these effects. It is recommended that patients eat a snack and drink sufficient fluid (such as water, milk, or juice) about 30 minutes before the injection.
Children and adolescents
There is not enough information about the use of this medicine in children under 4 months, and therefore, its use is not recommended.
Other medicines and Voxzogo
Tell your doctor if you or your child are taking, have recently taken, or might take any other medicines.
Pregnancy and breastfeeding
If you or your daughter are receiving treatment with this medicine and you or your daughter are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor before using this medicine.
The use of this medicine is not recommended during pregnancy and breastfeeding.
Driving, cycling, and using machines
This medicine may make you feel dizzy, tired, or nauseous for a few minutes after receiving the injection. In this case, do not drive, cycle, engage in physical activities, or use machines for about 1 hour after the injection or until you feel better.
Voxzogo contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per dose; this is essentially "sodium-free".
3. How to use Voxzogo
The Voxzogo injection should be administered by a caregiver. Do not inject Voxzogo into your child until you have received the necessary training from a healthcare professional.
Follow the instructions for administration of this medicine exactly as told by your doctor. If you are unsure, consult your doctor or pharmacist again.
Dose
Your doctor will choose the correct dose based on your or your child's body weight. The doctor will tell you how much solution for injection to inject. If you are unsure, consult your doctor or pharmacist.
Table 1 shows the dose that you or your child will need to inject daily based on body weight. The amount to inject will be presented in different volumes depending on the type of syringe included in your pack (milliliters (ml) or units (U)). Check that you have the correct dose for the syringe you are using.
Table 1: Single-dose volumes by body weight in ml and unit volumes (U)
Weight (kg) | Dose (mg) | Vosoritide 0.4 mg solvent (water for injectable preparations): 0.5 ml concentration: 0.8 mg/ml | Vosoritide 0.56 mg solvent (water for injectable preparations): 0.7 ml concentration: 0.8 mg/ml | Vosoritide 1.2 mg solvent (water for injectable preparations): 0.6 ml concentration: 2 mg/ml | |||
Daily injection volume | |||||||
ml | Units | ml | Units | ml | Units | ||
4 | 0.12 mg | 0.15 ml | 15 U | ||||
5 | 0.16 mg | 0.20 ml | 20 U | ||||
6-7 | 0.20 mg | 0.25 ml | 25 U | ||||
8-11 | 0.24 mg | 0.30 ml | 30 U | ||||
12-16 | 0.28 mg | 0.35 ml | 35 U | ||||
17-21 | 0.32 mg | 0.40 ml | 40 U | ||||
22-32 | 0.40 mg | 0.50 ml | 50 U | ||||
33-43 | 0.50 mg | 0.25 ml | 25 U | ||||
44-59 | 0.60 mg | 0.30 ml | 30 U | ||||
60-89 | 0.70 mg | 0.35 ml | 35 U | ||||
≥ 90 | 0.80 mg | 0.40 ml | 40 U |
You or your child should eat a snack and drink sufficient water, milk, or juice about 30 minutes before receiving the injection. This may reduce side effects such as dizziness, tiredness, or nausea.
How to use Voxzogo
Inject Voxzogo slowly under the skin (subcutaneous injection).
The injection should be administered at approximately the same time every day.
It is recommended to administer the injection in a different place each day and not to use the same place 2 days in a row. Do not inject the medicine into areas with moles, scars, or birthmarks, or into areas where the skin is sensitive, bruised, red, or hard.
If you use more Voxzogo than you should
If you inject more Voxzogo than you should, contact your doctor immediately.
If you forget to use Voxzogo
If your child misses a dose, they should receive the injection if it is not more than 12 hours since the scheduled time. If more than 12 hours have passed since the scheduled time, do not give the missed dose. Wait until the next day and continue with the usual dose at the usual time.
If you stop using Voxzogo
Before deciding to stop your child's treatment, always consult with your child's doctor. If you or your child have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Very common side effects
May affect more than 1 in 10 people:
- Vomiting
- Low blood pressure (temporary effects include dizziness, tiredness, or nausea shortly after receiving the injection)
- Injection site reactions: redness, itching, inflammation, swelling, bruising, rash, hives, pain. Injection site reactions are usually mild and resolve on their own within a few hours.
- High levels of alkaline phosphatase in the blood (shown in blood tests)
Common side effects
May affect up to 1 in 10 people:
- Nausea
- Feeling of dizziness and loss of consciousness
- Dizziness
- Tiredness
Reporting of side effects
If you or your child experience any side effects, talk to your doctor or nurse, even if they are not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Voxzogo
Keep this medicine out of the sight and reach of children.
Do notuse this medicine after the expiry date which is stated on the carton after "EXP". The expiry date is the last day of the month shown.
Store in a refrigerator (between 2°C and 8°C). Do not freeze.Keep in the original packaging to protect from light.
Voxzogo can be stored at room temperature (below 30°C) for up to 90 days, but not after the expiry date. Do notreturn Voxzogo to the refrigerator after it has been stored at room temperature. Writethe date on the carton when you remove Voxzogo from the refrigerator to store at room temperature.
Use Voxzogo as soon as it is prepared as a solution. In any case, it must be administered within 3 hours of preparation. Do not use this medicine if the solution for injection is cloudy or contains particles.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
Voxzogo Composition
- The active ingredient is vosoritide.
- Each 0.4 mg vial of reconstituted powder in a 0.5 ml solvent solution corresponds to a concentration of 0.8 mg/ml.
- Each 0.56 mg vial of reconstituted powder in a 0.7 ml solvent solution corresponds to a concentration of 0.8 mg/ml.
- Each 1.2 mg vial of reconstituted powder in a 0.6 ml solvent solution corresponds to a concentration of 2 mg/ml.
- The other components are citric acid (E 330), sodium citrate (E 331), trehalose dihydrate, mannitol (E 421), methionine, and polysorbate 80 (E 433).
- The solvent is water for injectable preparations.
Appearance of Voxzogo and Container Contents
Voxzogo powder and solvent for injectable solution are supplied as:
- a powder for injectable solution, white to yellow in color, in a glass vial and
- a clear and colorless solvent (water for injectable preparations) to dissolve the powder.
After dissolving the powder in the solvent, the solution is a clear, colorless to yellow liquid.
Each box contains:
- 10 Voxzogo vials
- 10 prefilled syringes with water for injectable preparations
- 10 single-use needles
- 10 single-use syringes
Marketing Authorization Holder and Manufacturer
BioMarin International Limited
Shanbally, Ringaskiddy
County Cork
Ireland
P43 R298
Date of Last Revision of this Leaflet: MM/YYYY.
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu. There are also links to other websites on rare diseases and orphan medicines.
Instructions for Use of the Milliliter (ml) Graduated Syringe
Read these instructions for use before using Voxzogo and each time you perform a refill. There may be new information.
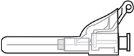
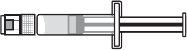
Supplied Items for Injecting Voxzogo (see Figure A)
Figure A
Voxzogo Vial | Solvent Needle (the blue tab retracts the needle) | Solvent Syringe(contains water for injectable preparations for the reconstitution of Voxzogo) |
|
|
|
Injectable Syringe

Consult your doctor or healthcare professional if you are unsure of the recommended dose or how to use the solvent needle and injectable syringe.
Items Needed but Not Supplied in the Container (see Figure B)
If you do not have these items, consult your pharmacist.
Figure B
Alcohol Swabs | Sharps Container | Gauze or Dressings |
|
|
|
PREPARATION FOR INJECTION
Before starting, ensure that the work surface is clean and that you have washed your hands.
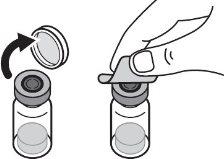
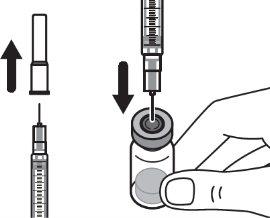
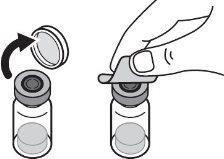
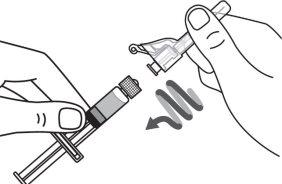
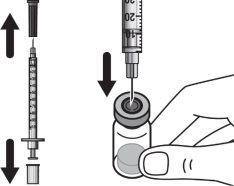
Step 1:On a clean and flat surface, remove the closure cap from the vial and clean the top with an alcohol swab. Do not touch the vial stopper with your fingers after cleaning it with the alcohol swab. |
|
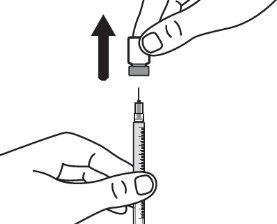
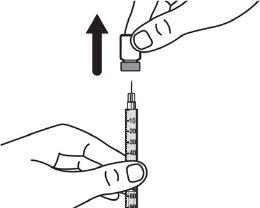
Step 2:Gently tilt the solvent syringe to remove the cap. |
|
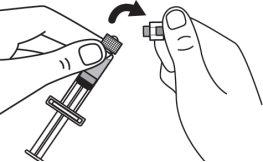
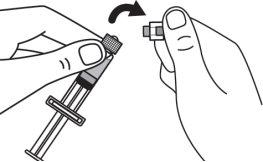
Step 3:Screw the solvent needle onto the solvent syringe until it can no longer be turned. |
|
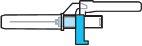
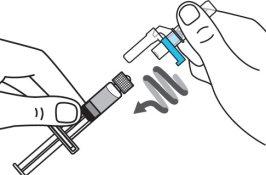
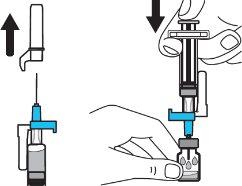
Step 4:Remove the needle cap and insert the needle into the vial through the middle of the stopper. Slowly push the solvent syringe plunger to inject all the liquid. Be careful not to push the blue tab until step 5. |
|
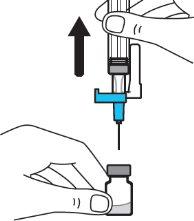
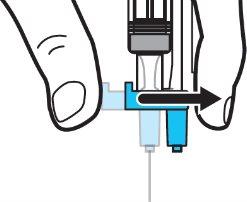
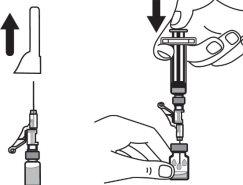
Step 5:Remove the needle from the vial and then press the blue tab to retract the needle. Discard the needle and syringe in a sharps container. See step 19 and “How to Dispose of (Eliminate) Voxzogo”. Do not use the solvent syringe to administer the injection. CAUTION: Be careful not to touch the needle tip. |
|
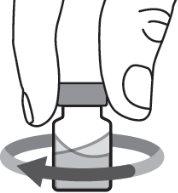
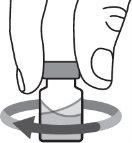
Step 6:Gently swirl the vial in a circular motion until the powder is completely dissolved and the solution is clear. Do not shake the vial. Make sure the medication has a color of clear to yellow and is not cloudy or contains particles. |
|
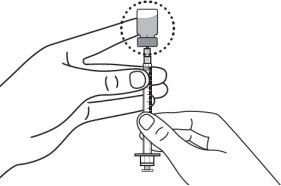
Step 7:Remove the needle cap from the injectable syringe and insert the needle into the vial in a straight line through the middle of the stopper. Be careful not to bend the needle. CAUTION: Do not replace the needle cap. |
|
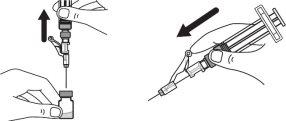
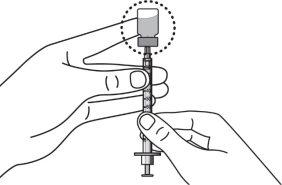
Step 8:Carefully hold the vial and syringe and invert the vial with the needle still inserted. The vial should be on top. Be careful not to bend the needle. |
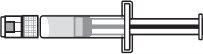
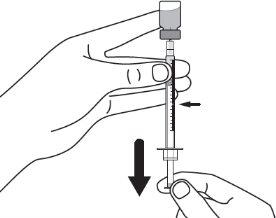
|
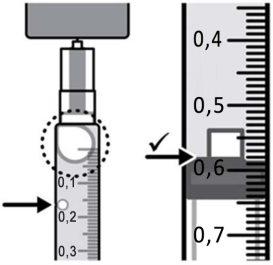
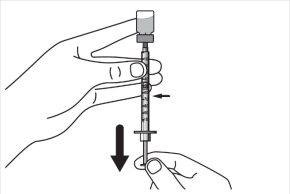
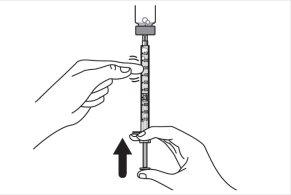
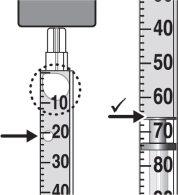
Step 9:Keep the needle tip in the medication and slowly pull the syringe plunger back to withdraw the prescribed dose into the syringe. Check the prescription label for the amount to be withdrawn. CAUTION: Withdraw the prescribed dose. |
|
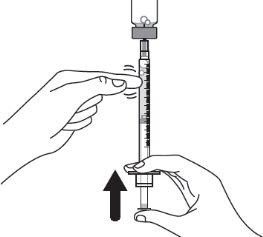
Step 10:Gently tap the syringe with a finger to remove large air bubbles. Then, slowly push the air bubbles back into the vial. |
|
Step 11:Repeat steps 9 and 10 until you have the correct prescribed dose in the syringe and there are no large air bubbles. Make sure the dose in the syringe matches the prescribed dose. Measure from the base of the plunger, as shown in the figure. CAUTION: Remove large air bubbles. It is acceptable to have 1 or 2 small air bubbles. |
|
Step 12:Make sure you have the prescribed dose in the syringe and then remove the vial and prepare to administer the dose. CAUTION:Before removing the vial, check that the amount matches the prescribed dose. |
|
SELECTION AND PREPARATION OF THE INJECTION SITE
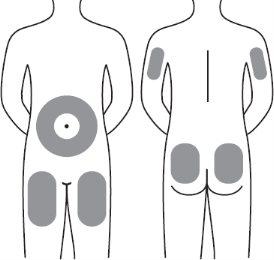
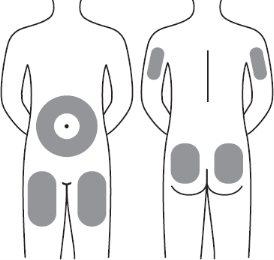
Step 13:Voxzogo should be injected into the fat layer under the skin (subcutaneous layer) only.
| It is recommended to use the following areas for injections:
|
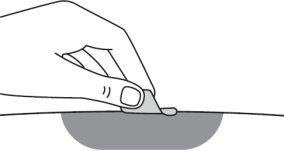
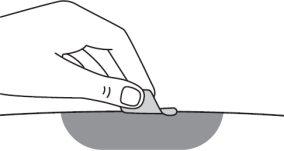
Step 14:Clean the injection site with an alcohol swab and let the skin air dry. Do not touch the injection site again before administering the injection. |
|
ADMINISTRATION OF THE VOXZOGO INJECTION
Step 15:After cleaning the area with an alcohol swab, pinch the skin up around the chosen injection site. |
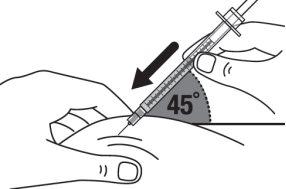
|
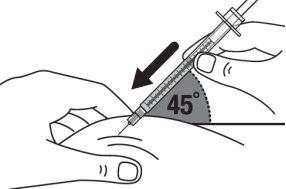
Step 16:Quickly insert the entire needle into the skin at a 45-degree angle. |
|
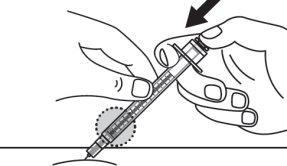
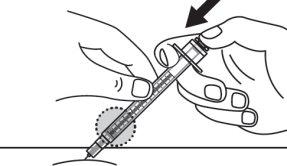
Step 17:Release the skin pinch and slowly push the syringe plunger all the way in. Inject the full dose. |
|
Step 18:Continue pressing the plunger until the needle retracts into the syringe. |
|
Step 19:Discard the used vial, syringes, and needles in a sharps container. See “How to Dispose of (Eliminate) Voxzogo” for more information. |
|
AFTER INJECTING VOXZOGO
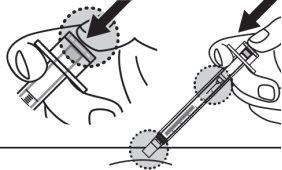
- Check the injection site. If you notice a small amount of blood at the injection site, gently press with a gauze for a few seconds or apply a dressing.
- Do notrub the injection site.
- Be aware of signs of low blood pressure, such as dizziness, fatigue, or feeling unwell. If you have these symptoms, call your doctor or healthcare professional and then lie down on your back and place pillows under your legs to keep them elevated.
HOW TO DISPOSE OF (ELIMINATE) VOXZOGO
Place used vials, needles, and syringes in a sharps container immediately after use.
If you do not have a sharps container, you can use a household container that:
- is made of heavy-duty plastic;
- can be closed with a tight-fitting, puncture-resistant lid that prevents the sharps from coming out;
- is kept upright and stable during use;
- is leak-resistant and
- is properly labeled to warn that it contains hazardous waste.
When the container you use for disposing of sharps is almost full, you should follow local guidelines for disposing of it properly.
Medicines, vials, loose needles, and syringes should not be thrown away in the trash. Ask your pharmacist how to dispose of the packaging and medicines you no longer need. This will help protect the environment.
INSTRUCTIONS FOR USE OF THE UNIT (U) GRADUATED SYRINGE
The solvent needles and administration syringes included in this package are new components marked with “Units” (U) to measure the recommended dose. Your doctor will indicate the recommended dose to administer based on your weight interval.
Supplied Items for Injecting Voxzogo (see Figure A)
Figure A
Voxzogo Vial | Solvent Needle | Solvent Syringe(contains water for injectable preparations for the reconstitution of Voxzogo) |
|
|
|
Injectable Syringe

Your dose can be administered using the injectable syringe in Figure A. The measurements for this syringe are equivalent to ml as follows: 0.1 ml = 10 units.
Items Needed but Not Supplied in the Container (see Figure B)
If you do not have these items, consult your pharmacist.
Figure B
Alcohol Swabs | Sharps Container | Gauze or Dressings |
|
|
|
PREPARATION FOR INJECTION
Before starting, ensure that the work surface is clean and that you have washed your hands.
Step 1:On a clean and flat surface, remove the closure cap from the vial and clean the top with an alcohol swab. Do not touch the vial stopper with your fingers after cleaning it with the alcohol swab. |
|
Step 2:Gently tilt the solvent syringe to remove the cap. |
|
Step 3:Screw the solvent needle onto the solvent syringe until it can no longer be turned. |
|
Step 4:Remove the needle cap and insert the needle into the vial through the middle of the stopper. Slowly push the solvent syringe plunger to inject all the liquid. |
|
Step 5:Remove the needle from the vial. Discard the needle and syringe in a sharps container. See step 18 and “How to Dispose of (Eliminate) Voxzogo”. Do not use the solvent syringe to administer the injection. CAUTION: Be careful not to touch the needle tip. |
|
Step 6:Gently swirl the vial in a circular motion until the powder is completely dissolved and the solution is clear. Do not shake the vial. Make sure the medication has a color of clear to yellow and is not cloudy or contains particles. |
|
| |
Step 7:Remove the needle cap from the syringe for injectables and insert the needle into the vial in a straight line through the middle of the stopper. Be careful not to bend the needle. WARNING: Do not put the cap back on the needle. |
|
Step 8:Carefully hold the vial and syringe and invert the vial with the needle still inserted. The vial should be on top. Be careful not to bend the needle. |
|
Step 9:Keep the tip of the needle in the medication and, slowly, pull the plunger back to extract the prescribed dose into the syringe. Check the prescription label for the amount to be extracted. WARNING: Verify the syringe supplied in the packaging and extract the prescribed dose. |
|
Step 10:Gently tap the syringe with a finger to eliminate large air bubbles. Then, slowly push the bubbles back into the vial. |
|
Step 11:Repeat steps 9 and 10 until you have the correct prescribed dose in the syringe and there are no large bubbles. Make sure the dose in the syringe matches the prescribed dose. Measure from the base of the plunger, as shown in the figure. WARNING: Eliminate large bubbles. It is acceptable to have 1 or 2 small bubbles. |
|
Step 12:Make sure you have the prescribed dose in the syringe and then remove the vial and prepare to administer the dose. WARNING:Before removing the vial, check that the amount matches the prescribed dose. |
|
SELECTION AND PREPARATION OF THE INJECTION SITE
Step 13:Voxzogo should be injected into the fat layer under the skin (subcutaneous layer) only. Do not inject through clothing. Do not inject into the same area twice in a row. Do not inject into painful, bruised, red, hard, or scarred skin
| It is recommended to use the following areas for injections:
|
Step 14:Clean the injection site with an alcohol swab and let the skin dry on its own. Do not touch the area again before administering the injection. |
|
ADMINISTRATION OF THE VOXZOGO INJECTION
Step 15:After cleaning the area with an alcohol swab, pinch the skin upwards around the chosen injection site. |
|
Step 16:Quickly insert the entire needle into the skin at a 45-degree angle. |
|
Step 17:Stop pinching and, slowly, push the syringe plunger all the way in. Inject the full dose. |
|
Step 18:Dispose of the used vial, syringes, and needles in a sharps disposal container. See "How to dispose of (discard) Voxzogo" for more information. |
|
AFTER INJECTING VOXZOGO
- Check the injection site. If you see a small amount of blood at the injection site, gently press with a gauze for a few seconds or apply a bandage.
- Do notrub the injection site.
- Be aware of signs of low blood pressure, such as dizziness, fatigue, or feeling sick. If you have these symptoms, call your doctor or healthcare professional and then lie down on your back and put pillows under your legs to keep them elevated.
HOW TO DISCARD (DISPOSE OF) VOXZOGO
Place used vials, needles, and syringes in a sharps disposal container immediately after use.
If you do not have a sharps disposal container, you can use a household container that:
- is made of heavy-duty plastic;
- can be closed with a tight-fitting, puncture-resistant lid to prevent sharps from coming out;
- is kept upright and stable during use;
- is leak-resistant and
- is properly labeled to warn that it contains hazardous waste.
When the container you use to discard sharps is almost full, you should follow local guidelines for proper disposal.
Medicines, vials, loose needles, and syringes should not be thrown away in the trash. Ask your pharmacist how to dispose of containers and medicines you no longer need. This will help protect the environment.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to VOXZOGO 0.4 mg POWDER AND SOLVENT FOR INJECTABLE SOLUTIONDosage form: INJECTABLE, 0.56 mgActive substance: vosoritideManufacturer: Biomarin International LimitedPrescription requiredDosage form: INJECTABLE, 1.2 mgActive substance: vosoritideManufacturer: Biomarin International LimitedPrescription requiredDosage form: INJECTABLE, 120 mgActive substance: denosumabManufacturer: Fresenius Kabi Deutschland GmbhPrescription required
Online doctors for VOXZOGO 0.4 mg POWDER AND SOLVENT FOR INJECTABLE SOLUTION
Discuss questions about VOXZOGO 0.4 mg POWDER AND SOLVENT FOR INJECTABLE SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions