
VITAMIN D3 FRESENIUS KABI 14,400 IU/ML ORAL DROPS IN SOLUTION

How to use VITAMIN D3 FRESENIUS KABI 14,400 IU/ML ORAL DROPS IN SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Vitamin D3 Fresenius Kabi 14,400 IU/ml Oral Drops in EFG Solution
colecalciferol (vitamin D3)
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the Package Leaflet
- What is Vitamin D3 Fresenius Kabi and what is it used for
- What you need to know before you take Vitamin D3 Fresenius Kabi
- How to take Vitamin D3 Fresenius Kabi
- Possible side effects
- Storage of Vitamin D3 Fresenius Kabi
- Contents of the Pack and Further Information
1. What is Vitamin D3 Fresenius Kabi and what is it used for
This medicine contains the active substance colecalciferol (vitamin D3).
This medicine is used for:
- Prevention and treatment of vitamin D deficiency
- Treatment of rickets (a disease affecting bone development in children)
- As an adjunct to treatment for osteoporosis in patients at risk of vitamin D deficiency
2. What you need to know before you take Vitamin D3 Fresenius Kabi
Do not take Vitamin D3 Fresenius Kabi:
- if you are allergic to the active substance or any of the other ingredients of this medicine (listed in section 6).
- if you have any disease that causes high levels of calcium in the blood or increased excretion of calcium in the urine (such as with treatment with certain medications, such as benzothiazine derivatives, or if you are bedridden)
- if you have, or tend to develop, kidney stones containing calcium
- if you have an excess of vitamin D
- if you have severe arteriosclerosis (hardening of the arteries)
- if you have severe kidney dysfunction
Warnings and Precautions
Consult your doctor or pharmacist before taking this medicine.
You should be particularly careful when taking this medicine:
- if you are being treated with certain heart medications (cardiac glycosides) or thiazide diuretics
- if you have severe kidney dysfunction. In this case, your doctor will monitor your blood calcium and phosphate levels. The risk of soft tissue calcification should be taken into account.
- in case of severe renal dysfunction, the use of colecalciferol is not recommended. Your doctor may prescribe another vitamin D supplement.
- if you have sarcoidosis (Boeck's disease), there is a risk of increased formation of the active form of vitamin D.
- in case of decreased bone mass due to inactivity (e.g., bed rest), there is a higher risk of elevated blood calcium levels.
During long-term use, your doctor will periodically check your blood calcium levels and kidney function, and control your urine calcium levels. If necessary, your doctor will instruct you to reduce the dose or discontinue treatment.
Increased levels of parathyroid hormone may increase vitamin D metabolism and thus increase vitamin D requirements.
Additional doses of vitamin D should only be taken under close medical supervision.
Children and Adolescents
The use of other products containing vitamin D should be avoided, especially in infants. In case of doubt, the doctor will decide on the suitability of additional use of vitamin-enriched foods or medications containing vitamin D.
Other Medicines and Vitamin D3 Fresenius Kabi
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines. This is especially important if you are taking:
- Rifampicin or isoniazid (for tuberculosis).
- Certain heart medications (cardiac glycosides), as they may potentiate the harmful effects by increasing blood calcium levels (risk of arrhythmias). Close medical supervision is necessary, which may include an electrocardiogram (ECG) and monitoring of blood calcium levels.
- Thiazide diuretics, as they increase the risk of elevated blood calcium levels, since these medications reduce calcium excretion in the urine. In this case, your doctor will periodically check your blood calcium levels.
- Medications for the treatment of epilepsy (carbamazepine, phenobarbital, phenytoin, primidone) or certain corticosteroids (glucocorticoids, cortisone), as they may increase vitamin D requirements.
- Medications to reduce blood lipids (e.g., orlistat and cholestyramine), as they may reduce vitamin D absorption in the intestine.
- Medications containing magnesium (e.g., antacids), which should not be used during treatment with this medicine, as they may increase blood magnesium levels (hypermagnesemia).
- Aluminum-containing tablets (for heartburn).
Taking Vitamin D3 Fresenius Kabi with Food and Drinks
Caution is recommended with vitamin-enriched foods or infant formulas.
Pregnancy and Breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Pregnancy
If you are pregnant and your doctor prescribes this medicine, you should strictly follow the dose prescribed by your doctor, as an overdose of vitamin D3 may pose a risk of physical and mental retardation, as well as heart and eye diseases, in your baby.
Breastfeeding
Vitamin D and its metabolites pass into breast milk. No overdose-induced symptoms have been observed in breastfed infants. However, this should be taken into account if the baby receives additional vitamin D.
Driving and Using Machines
The influence of this medicine on the ability to drive and use machines is negligible.
3. How to Take Vitamin D3 Fresenius Kabi
Follow exactly the administration instructions of this medicine given by your doctor or pharmacist. If you are unsure, consult your doctor or pharmacist again.
Your doctor will determine the suitable dose for you. The dose depends on the disease for which you are prescribed this medicine. Therefore, always take the dose that has been prescribed for you individually, even if you know people who take a higher dose.
Unless your doctor tells you otherwise, the recommended dose is as follows:
Prevention of Vitamin D Deficiency:
The usual daily dose is:
For newborns, infants, and children from the second week of life to 3 years of age: 1 to 2 drops.
For children from 4 years of age and adolescents: 1 to 3 drops.
For adults from 19 to 70 years of age: 1 to 4 drops.
For elderly over 70 years of age: 2 to 4 drops.
Treatment of Rickets
The total amount of vitamin D required depends on the severity of the disease.
In case of pre-existing rickets, treatment is initiated with a preparation at a higher concentration of vitamin D for initial treatment.
Afterwards, the usual dose is 2 to 12 drops of this medicine per day.
Treatment of Vitamin D Deficiency
The usual daily dose is:
For children and adolescents: 5 drops per day for 6 weeks, and then 1 to 3 drops per day.
For adults from 19 to 70 years of age, and elderly over 70 years of age: 15 drops per day for 8 weeks, and then 3 to 5 drops per day.
As an Adjunct to Treatment for Osteoporosis in Patients at Risk of Vitamin D Deficiency
The usual daily dose is:
For adults from 19 years of age onwards: 2 to 4 drops per day, or 14 to 26 drops per week.
Method of Administration
This medicine should be taken orally. The best way is to add it drop by drop to the mouth or, if necessary, administer it with a spoon and some liquid.
If you feel that the effect of this medicine is too strong or too weak, consult your doctor or pharmacist.
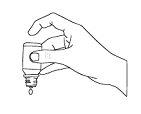
Instructions for Use
For administration, hold the bottle in a vertical position and gently tap the base of the bottle with your finger until the first drop comes out.
If You Take More Vitamin D3 Fresenius Kabi Than You Should
In case of overdose with this medicine, contact a doctor.
Symptoms of overdose may include: headache, loss of appetite, weakness, weight loss, digestive disorders (nausea, vomiting, constipation), growth disorders, increased urine output, increased fluid intake, signs of paralysis, weakness, increased blood pressure, and in severe cases, irregular heartbeat.
Your doctor will assess the severity of the poisoning and determine the necessary treatment.
If You Forget to Take Vitamin D3 Fresenius Kabi
Do not take a double dose to make up for forgotten doses.
If You Stop Taking Vitamin D3 Fresenius Kabi
No withdrawal symptoms have been observed.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible Side Effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Vitamin D may cause the following side effects, especially in case of overdose:
Metabolic and Nutritional Disorders:
High levels of calcium in the blood and urine (hypercalcemia, hypercalciuria).
Gastrointestinal Disorders:
Constipation, bloating, nausea, stomach pain, diarrhea.
Frequency of occurrence of the above-mentioned side effects not known (cannot be estimated from the available data).
Reporting of Side Effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly to the Spanish Medicines and Healthcare Products Agency (AEMPS) at www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Vitamin D3 Fresenius Kabi
- Do not store above 30°C.
- Keep the bottle in the outer carton to protect it from light.
- Keep this medicine out of the sight and reach of children.
- Do not use this medicine after the expiry date which is stated on the label and carton after EXP. The expiry date is the last day of the month stated.
- After first opening, use this medicine within 10 months. Any remaining product should be discarded. Do not store above 25°C after first opening.
- Do not use this medicine if you notice: turbidity or discoloration of the solution.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Contents of the Pack and Further Information
Composition of Vitamin D3 Fresenius Kabi
- The active substance is colecalciferol (vitamin D3).
1 ml (= 36 drops) contains:
14,400 IU (360 µg) of colecalciferol (vitamin D3)
1 drop contains 400 IU (10 µg) of colecalciferol (vitamin D3)
- The other ingredients are:
Medium-chain triglycerides
Appearance and Packaging of the Product
Vitamin D3 Fresenius Kabi is presented as clear to slightly yellowish oral drops (in oily solution) and is available in brown glass bottles with a dropper applicator and a screw cap with a polyethylene tamper-evident ring, containing 12.5 ml (corresponding to 450 drops) or 25 ml (corresponding to 900 drops).
Not all pack sizes may be marketed.
Marketing Authorisation Holder
Fresenius Kabi España S.A.U.
C/ Marina 16-18.
08005 Barcelona (Spain)
Manufacturer
Fresenius Kabi Austria GmbH
Estermannstraße 17
A-4020 Linz
Austria
This medicine is authorised in the Member States of the European Economic Area under the following names:
Austria: Colecalciferol Fresenius 14,400 I.E./ml Tropfen zum Einnehmen, Lösung
Belgium: Kabivit-D3 14400 IE/ml, druppels voor oraal gebruik, oplossing
Kabivit-D3 14400 IE/ml, Tropfen zum Einnehmen, Lösung
Kabivit-D3 14400 UI/ml, solution buvable en gouttes
Bulgaria: ??????? D3 14 400 IU/ml ????????? ?????, ???????
Cyprus: KabiVit 14.400 IU/ml, ?????? ?????
Slovakia: Olvit D3, 14 400 IU/ml perorálne roztokové kvapky
Slovenia: Oleovit D3 14400 i.e./ml peroralne kapljice, raztopina
Spain: Vitamin D3 Fresenius Kabi 14,400 IU/ml gotas orales en solución EFG
Estonia: Colecalciferol Fresenius
Finland: Oleo D3 14 400 IU/ml tipat, liuos
Greece: KabiVit 14.400 IU/ml
Hungary: Vitamin D3 Fresenius Kabi 14000 NE/ml belsoleges oldatos cseppek
Ireland: Sapvit-D3 400 IU/drop Oral Drops, Solution
Latvia: Colecalciferol Fresenius 14400 SV/ml pilieni iekškigai lietošanai, škidums
Lithuania: Colecalciferol Fresenius 14400 TV/ml geriamieji lašai (tirpalas)
Malta: KabiVit 14.400 IU/ml oral drops, solution
Norway: KabiVit
Poland: OlVit D3
Portugal: Colecalciferol Kabi
United Kingdom: Sapvit-D3 14,400 IU/ml Oral Drops, Solution
Czech Republic: Olvit D3 14400 IU/ml perorální kapky, roztok
Romania: Vitamina D3 Fresenius Kabi 14 400 UI/ml picaturi orale, solutie
Sweden: Oleo D3 14 400 IE/ml orala droppar, lösning
Date of Last Revision of this Leaflet:July 2021
Detailed information on this medicine is available on the website of the Spanish Agency for Medicines and Healthcare Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price16.86 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to VITAMIN D3 FRESENIUS KABI 14,400 IU/ML ORAL DROPS IN SOLUTIONDosage form: CAPSULE, 25,000 IUActive substance: colecalciferolManufacturer: Laboratorios Alter S.A.Prescription requiredDosage form: CAPSULE, 50,000 IUActive substance: colecalciferolManufacturer: Laboratorios Alter S.A.Prescription requiredDosage form: CAPSULE, 50,000 IUActive substance: colecalciferolManufacturer: Consilient Health LimitedPrescription required
Online doctors for VITAMIN D3 FRESENIUS KABI 14,400 IU/ML ORAL DROPS IN SOLUTION
Discuss questions about VITAMIN D3 FRESENIUS KABI 14,400 IU/ML ORAL DROPS IN SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















