
BCG Vaccine 0.75 mg/ml Powder and Diluent for Suspension for Injection

How to use BCG Vaccine 0.75 mg/ml Powder and Diluent for Suspension for Injection
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
PACKAGE LEAFLET: INFORMATION FOR THE USER
BCG Vaccine 0.75 mg/ml powder and solvent for injectable suspension
Mycobacterium bovis
Read all of this leaflet carefully before you or your child are vaccinated, as it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you or your child only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is BCG Vaccine and what is it used for
- What you need to know before you or your child receive BCG Vaccine
- How BCG Vaccine is administered
- Possible side effects
- Storage of BCG Vaccine
- Contents of the pack and other information
1. What is BCG Vaccine and what is it used for
BCG Vaccine powder and solvent for injectable suspension belongs to a group of medicines called antitubercular vaccines.
BCG Vaccine is indicated for the prevention of tuberculosis. Although it does not ensure complete immunity, it increases resistance to tubercular infection.
BCG Vaccine should be used based on official recommendations.
2. What you need to know before you or your child receive BCG Vaccine
Do not useBCG Vaccine
- If you are allergic (hypersensitive) to Mycobacterium bovisor any of the other components of BCG Vaccine (listed in section 6).
- If you have tuberculosis or any other infectious disease (active or during convalescence) or if you are undergoing antitubercular treatment.
- If you have any immune system disorders, particularly in patients with HIV infection, in children born to seropositive mothers, in cases of congenital immunodeficiency, or cases with diminished immune response due to certain medications (corticosteroids, alkylating agents, antimetabolites) or radiation.
- If you have been exposed to immunosuppressive treatment in the womb or during breastfeeding (e.g., treatment with an α-FNT antagonist).
- If your immune status is questionable.
- In children with Kwashiorkor malnutrition (which occurs with protein and calorie deficiency).
- If you have a severe acute febrile syndrome or any generalized skin disease (vaccination should be postponed).
- If you have severe angiopathies (blood vessel diseases) or hemopathies (blood diseases).
- If you have oncological processes.
Warnings and precautions
Consult your doctor or pharmacist before you or your child are vaccinated with BCG Vaccine.
Before starting treatment with BCG Vaccine, you should undergo a tuberculin test. Up to the age of eight, skin tests can be used, but in older children or adults, the intracutaneous Mantoux test with tuberculin should be used.
Although allergic reactions are rare, you should have the necessary measures in place for their treatment, and if possible, it is recommended to observe the patient for 15-20 minutes after injection for symptoms of an allergic reaction.
In case the patient has eczema, BCG Vaccine injection is not contraindicated, but the injection should be performed in a lesion-free area.
Use ofBCG Vaccine withother medicines
Tell your doctor or pharmacist if you or your child are using, have recently used, or might use any other medicines.
BCG Vaccine should not be administered to patients who have been treated with antitubercular medications.
BCG Vaccine can be administered at the same time as live vaccines, including combined vaccines (measles, mumps, and rubella), taking special precautions not to administer them in the same arm. If they are not administered simultaneously, a minimum interval of 4 weeks should be left between the administration of the two live vaccines.
To avoid the risk of swelling and pain in the lymph nodes of the area, it is recommended not to use the same arm where BCG Vaccine was applied for the administration of other vaccines for a period of three months.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before being vaccinated with BCG Vaccine.
Although no harm to the fetus has been associated with the use of BCG Vaccine, its administration is not recommended during pregnancy or breastfeeding, unless there is an excessive or unavoidable risk of exposure to tuberculosis.
Consult your doctor or pharmacist before using any medicine.
Driving and using machines
BCG Vaccine does not affect your ability to drive or use machines.
BCG Vaccine contains sodium
This medicine contains less than 23 mg of sodium per dose; this is essentially "sodium-free".
3. How BCG Vaccine is administered
Follow the administration instructions of BCG Vaccine exactly as indicated by your doctor. If in doubt, consult your doctor or pharmacist again.
The recommended dose is:
Adults and children over 1 year: a single dose of 0.1 ml.
Children under 1 year: a single dose of 0.05 ml.
Method of use and route of administration
BCG Vaccine is administered strictly by the INTRADERMAL route on the upper outer aspect of the arm and on the outer aspect of the thigh. The injection should be performed slowly in the upper layer of the skin by trained personnel, as if the injection is performed more deeply, the risk of abscess formation (localized accumulation of pus in the skin) increases.
If you use moreBCG Vaccinethan you should
In cases of overdose, especially in small children, suppurative benign lymphadenitis (inflammation of the lymph nodes) may occur, which heals slowly and spontaneously.
In exceptional cases, a generalized infection by BCG Vaccine may develop. Advice should be sought regarding the treatment regimen for the management of systemic or persistent local infections after vaccination with BCG Vaccine.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service (Telephone: 91 562 04 20) indicating the medicine and the amount ingested.
If you miss the administration of BCGVaccine
As a single dose, it is unlikely that you will miss your dose. However, inform your doctor or pharmacist if you have missed your dose.
4. Possible side effects
Like all medicines, BCG Vaccine can cause side effects, although not everybody gets them.
Generally, this vaccination does not usually cause fever or discomfort. A few days after vaccination, a nodule of induration (hardened tissue swelling that forms in the skin) develops at the injection site. This nodule gradually decreases and is replaced by a local lesion that may ulcerate some weeks later. The local lesion does not require treatment, and dressings should not be used. This lesion heals spontaneously with the formation of a small black scab.
Occasionally, an enlargement of the lymph nodes, cervical or axillary, may be observed, which also does not require treatment.
The following adverse reactions have been observed, classified by organ and system, in decreasing order of frequency:
Uncommon side effects (at least 1 in 1,000 patients):
Enlargement of lymph nodes (> 1 cm), headache, fever, ulceration at the injection site, inflammation with pus of the lymph nodes.
Rare side effects (at least 1 in 10,000 patients):
Disseminated infection, such as acute or chronic inflammation of the bones, caused or not by an infection, abscess at the injection site, allergic reaction, hypersensitivity reaction.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of BCG Vaccine
Store in a refrigerator (between 2°C and 8°C) protected from light.
Use only within 4 hours after reconstitution. After this period, discard the suspension.
Keep this medicine out of the sight and reach of children.
Do not use BCG Vaccine after the expiry date stated on the packaging. The expiry date is the last day of the month indicated.
Medicines should not be disposed of via wastewater or household waste. Return the packaging and any unused medicine to the pharmacy. If in doubt, ask your pharmacist how to dispose of the packaging and any unused medicine. This will help protect the environment.
6. Contents of the pack and other information
Composition of BCG Vaccine
- The active ingredient of BCG Vaccine is Mycobacterium bovis(BCG) Danish strain 1331. Each 1 ml of reconstituted vaccine contains 0.75 mg of Mycobacterium bovis(BCG) Danish strain 1331, with 2-8 x 10^6 CFU/ml.
- The other components are: sodium glutamate, magnesium sulfate, potassium dibasic phosphate, L-asparagine monohydrate, ferric ammonium citrate, glycerol 85%, citric acid monohydrate, and water for injectable preparations, q.s.
Appearance of the product and contents of the pack
BCG Vaccine is presented as a powder and solvent for injectable suspension.
The powder is a white crystalline lyophilizate, barely perceptible to the eye due to the small amount contained in the vial. The powder is packaged in a type I glass vial with a bromobutyl stopper and an aluminum cap.
The solvent is a clear, particle-free solution. The solvent is packaged in a type I glass vial with a chlorobutyl stopper and an aluminum cap.
A vial of reconstituted BCG Vaccine contains 1 ml, corresponding to 10 doses for adults and children over 1 year (0.1 ml) or 20 doses for children under 1 year (0.05 ml).
Marketing Authorization Holder and Manufacturer
AJ Vaccines A/S, Artillerivej 5, DK-2300 Copenhagen S, Denmark.
You can request more information about this medicine by contacting the local representative of the Marketing Authorization Holder:
MEDICARE PHARMA, S.L.
Paseo de la Castellana, 177 3ºB, 28046 Madrid, Spain
This leaflet was approved in 06/2020
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
_____________________________________________________________________________________
This information is intended only for healthcare professionals:
Special warnings and precautions for use
The vaccine should be administered only by the intradermal route.
Preferably, the vaccine should be administered by trained personnel in the intradermal vaccination technique.
Inadequately administered injections, for example, subcutaneously or intramuscularly, increase the risk of lymphadenitis and abscess formation.
Do not vaccinate individuals who test positive for tuberculin, as this may worsen the local-regional reaction.
Although anaphylactic reactions are rare, facilities for their treatment should be available during vaccination.
Whenever possible, individuals should be kept under observation for 15-20 minutes after vaccination in case an allergic reaction occurs.
BCG Vaccine can be administered at the same time as inactivated or live vaccines, including combined vaccines against measles, mumps, and rubella. If they are not administered simultaneously, a minimum interval of 4 weeks should be left before administering another live vaccine.
A minimum interval of 3 months should be waited before placing a new vaccine in the same arm.
Handling
The rubber stopper should not be cleaned with any antiseptic or soap. If alcohol is used to clean the vial stopper, it should be allowed to evaporate before the needle of the syringe penetrates it.
Using a syringe equipped with a long needle, transfer the specified volume of solvent to the vial.
Do not use other solvents, as they may damage the vaccine.
Gently invert the vial several times to completely resuspend the lyophilizate.
DO NOT SHAKE. Before extracting each dose of reconstituted vaccine, gently shake the vial.
When extracted into the syringe, the vaccine suspension should appear homogeneous, slightly opaque, and colorless.
Once reconstituted, the vaccine should be used within 4 hours.
Method of administration
BCG Vaccine should be administered by personnel trained in the intradermal technique.
The area where the injection will be applied should be clean and dry.
If an antiseptic (e.g., alcohol) is used to clean the skin, it should be allowed to evaporate completely before injection.
BCG Vaccine is administered strictly by the INTRADERMAL route in the upper third of the arm corresponding to the area of distal insertion of the deltoid muscle as follows:
- The skin should be stretched between the index finger and thumb.
- The needle should be almost parallel to the skin surface and inserted slowly (with the bevel up), approximately 2 mm into the superficial layer of the dermis. The needle should be visible through the epidermis during insertion.
- The injection should be performed slowly.
- If the administration is correct, a white papule will appear at the injection site.
- It is recommended not to protect the injection site to facilitate healing.
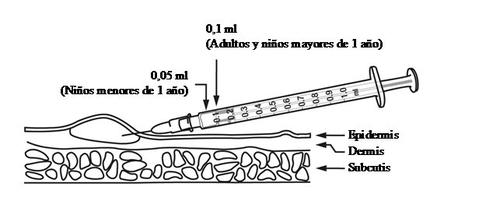
BCG Vaccine should be administered with a 1 ml syringe graduated in hundredths of a ml (1/100 ml) equipped with a short-bevel needle of 25G or 26G caliber. Jet injectors or multiple puncture devices should not be used for the administration of this vaccine.
Overdose or incorrect administration
An overdose increases the risk of suppurative lymphadenitis and may cause excessive scar formation.
A massive overdose increases the risk of BCG Vaccine side effects.
Deep injection of the vaccine increases the risk of suppurative ulcer, lymphadenitis, and abscess formation.
Treatment of complications after vaccination with BCG Vaccine
Advice should be sought regarding the appropriate treatment regimen for the management of systemic or persistent local infections after vaccination with BCG Vaccine.
Sensitivity of the BCG strain to antibiotics:
The table below indicates the minimum inhibitory concentration (MIC) values for the various antitubercular medications selected against the Danish strain 1331 [determined using the Bactec 460 method].
The MIC for isoniazid is 0.4 mg/l. There is no consensus on whether Mycobacterium bovis should be classified as susceptible, intermediate, or resistant to isoniazid when the MIC is 0.4 mg/l. However, based on the criteria established for Mycobacterium tuberculosis, the strain is considered to be of intermediate susceptibility.
Medicine | Minimum Inhibitory Concentration (MIC) |
Isoniazid | 0.4 mg/l |
Streptomycin | 2.0 mg/l |
Rifampicin | 2.0 mg/l |
Etambutol | 2.5 mg/l |
The Danish strain 1331 is resistant to pyrazinamide.
- Country of registration
- Average pharmacy price78.05 EUR
- Availability in pharmacies
Supply issue reported
Data from the Spanish Agency of Medicines (AEMPS) indicates a supply issue affecting this medicine.<br><br>Availability may be limited in some pharmacies.<br><br>For updates or alternatives, consult your pharmacist. - Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to BCG Vaccine 0.75 mg/ml Powder and Diluent for Suspension for InjectionDosage form: INJECTABLE, 0.5 ml single doseActive substance: meningococcus B, multicomponent vaccineManufacturer: Glaxosmithkline Vaccines S.R.L.Prescription requiredDosage form: INJECTABLE, 0.5 ml single doseActive substance: meningococcus B, multicomponent vaccineManufacturer: Glaxosmithkline Vaccines S.R.L.Prescription requiredDosage form: INJECTABLE, -Active substance: pertussis, purified antigen, combinations with toxoidsManufacturer: Glaxosmithkline S.A.Prescription required
Online doctors for BCG Vaccine 0.75 mg/ml Powder and Diluent for Suspension for Injection
Discuss questions about BCG Vaccine 0.75 mg/ml Powder and Diluent for Suspension for Injection, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















