TRAVOPROST/TIMOLOL STADA 40 micrograms/mL + 5 mg/mL Eye Drops Solution

How to use TRAVOPROST/TIMOLOL STADA 40 micrograms/mL + 5 mg/mL Eye Drops Solution
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Travoprost/Timolol Stada40 micrograms/ml+ 5 mg/ml eye drops, solution
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Travoprost/Timolol Stada and what is it used for
- What you need to know before you use Travoprost/Timolol Stada
- How to use Travoprost/Timolol Stada
- Possible side effects
- Storing Travoprost/Timolol Stada
- Contents of the pack and other information
1. What is Travoprost/Timolol Stada and what is it used for
Travoprost/Timolol Stada eye drops, solution is an association of two active substances (travoprost and timolol). Travoprost is a prostaglandin analogue that acts by increasing the outflow of aqueous fluid from the eye, thereby reducing the pressure in the eye. Timolol is a beta-blocker that acts by reducing the production of fluid in the eye. The two substances work together to reduce the pressure in the eye.
Travoprost/Timolol Stada eye drops are used to treat high pressure in the eyes in adults, including the elderly. This pressure can lead to a disease called glaucoma.
2. What you need to know before you use Travoprost/Timolol Stada
Do not use Travoprost/Timolol Stada
- if you are allergic to travoprost, prostaglandins, timolol, beta-blockers or any of the other ingredients of this medicine (listed in section 6).
- if you currently have or have a history of respiratory problems such as asthma, severe chronic obstructive pulmonary disease (a serious lung disease that can cause wheezing, difficulty breathing and/or persistent cough) or other types of respiratory problems.
- if you have severe allergic rhinitis.
- if you have a slow heart rate, heart failure or a heart rhythm disorder (irregular heartbeats).
- if the surface of your eye is cloudy.
Talk to your doctor if you are in any of these situations.
Warnings and precautions
Before starting to use this medicine, tell your doctor if you currently have or have a history of:
heart disease (symptoms may include chest pain, shortness of breath or choking), heart failure, low blood pressure,
- heart rhythm disorders such as slow heartbeat.
- respiratory problems, asthma or chronic obstructive pulmonary disease
- poor circulation (such as Raynaud's disease or Raynaud's syndrome)
- diabetes (as timolol may mask the signs and symptoms of low blood sugar)
- overactive thyroid gland (as timolol may mask the signs and symptoms of thyroid disease)
- myasthenia gravis (a chronic muscle weakness).
- cataract surgery
- eye inflammation
If you need to undergo any type of surgery, tell your doctor that you are using travoprost/timolol as timolol may affect the effects of some medicines used during anesthesia.
If you experience any severe allergic reaction (skin rash, redness and itching in the eye) while using travoprost/timolol, whatever the cause, treatment with adrenaline may not be as effective. It is therefore very important that you inform your doctor that you are using travoprost/timolol when you receive any other treatment.
Travoprost/timolol may change the color of your iris (the colored part of your eye). This change may be permanent.
Travoprost/timolol may increase the length, thickness, color and/or number of your eyelashes and may cause unusual hair growth on your eyelids.
Travoprost may be absorbed through the skin; therefore, pregnant or breastfeeding women should not handle this medicine. In case of contact with the skin, the medicine should be washed off immediately.
Children
Travoprost/timolol should not be used in children and adolescents under 18 years of age.
Other medicines and Travoprost/Timolol Stada
Tell your doctor or pharmacist if you are using, have recently used or might use any other medicines.
Travoprost/timolol may affect or be affected by other medicines you are using, including other eye drops for the treatment of glaucoma. Ask your doctor if you are using or plan to use:
- medicines to lower blood pressure,
- heart medicines including quinidine (used to treat heart conditions and some types of malaria),
- medicines to treat diabetes or antidepressants known as fluoxetine and paroxetine.
Pregnancy, breastfeeding and fertility
If you are pregnant or breastfeeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Do not use travoprost/timolol if you are pregnant unless your doctor considers it necessary. If you can become pregnant, you should use an effective method of birth control while using this medicine.
Do not use travoprost/timolol if you are breastfeeding. Travoprost/timolol may pass into breast milk.
Driving and using machines
Immediately after applying travoprost/timolol, you may notice that your vision becomes blurred. Do not drive or use machines until these effects have disappeared.
Travoprost/Timolol Stada contains benzalkonium chloride
This medicine contains 150 micrograms of benzalkonium chloride in each ml of eye drops, solution. Benzalkonium chloride may be absorbed by soft contact lenses and alter their color. Remove contact lenses before using this medicine and wait 15 minutes before putting them back.
Benzalkonium chloride may cause eye irritation, especially if you have dry eyes or other diseases of the cornea (the transparent layer on the front of the eye). Talk to your doctor if you feel any unusual sensation, itching or pain in the eye after using this medicine.
Travoprost/Timolol Stada contains macrogolglycerol hydroxystearate 40
This medicine may cause skin reactions because it contains macrogolglycerol hydroxystearate 40.
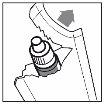
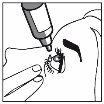
3. How to use Travoprost/Timolol Stada
Follow exactly the instructions of administration of this medicine given by your doctor. In case of doubt, consult your doctor or pharmacist again.
The recommended dose is
One drop in the affected eye(s), once a day - in the morning or in the evening. Use it at the same time each day.
Travoprost/timolol should only be applied in both eyes if your doctor has recommended it. Continue the treatment for the whole period of time indicated by your doctor.
This medicine should only be used as eye drops.
Instructions for use
1 |
|
2 |
|
3 |
|
4 |
|
If a drop falls outside the eye, try again.
If you use moreTravoprost/Timolol Stadathan you should
If you use more travoprost/timolol than you should, you may wash your eyes with warm water. Do not apply more drops until it is time for your next dose.
If you forget to useTravoprost/Timolol Stada
If you forget to use travoprost/timolol, continue with the next dose as planned. Do not apply a double dose to make up for the forgotten dose. The dose should not exceed 1 drop per day in the affected eye(s).
If you stop usingTravoprost/Timolol Stada
If you stop using travoprost/timolol without consulting your doctor, the pressure in your eye will not be controlled, which could lead to loss of vision.
If you are using another eye drop in addition to Travoprost/Timolol Stada, wait at least 5 minutes between applying travoprost/timolol and the other drops.
If you wear soft contact lenses, do not apply the drops while wearing them. After applying the drops, wait 15 minutes before putting your lenses back in.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Unless the effects are severe, continue with the treatment as usual. If these effects worry you, talk to your doctor or pharmacist. Do not stop using this medicine without talking to your doctor.
Very common side effects(may affect more than 1 in 10 people)
Eye effects:
Redness of the eye
Common side effects(may affect up to 1 in 10 people)
Eye effects:
Inflammation with damage to the surface of the eye, eye pain, blurred vision, abnormal vision, dry eye, itching of the eye, eye discomfort, signs and symptoms of irritation in the eye (e.g. burning, stinging).
Uncommon side effects(may affect up to 1 in 100 people)
Eye effects:
Inflammation of the surface of the eye, inflammation of the eyelid, swelling of the conjunctiva, increased growth of eyelashes, inflammation of the iris, inflammation of the eye, sensitivity to light, decreased vision, tired eyes, allergic eye reaction, swelling in the eye, increased tear production, redness of the eyelid, change in eyelid color, darkening of the skin (around the eye).
Other effects:
Allergic reaction to the active substance, dizziness, headache, increased or decreased blood pressure, shortness of breath, excessive hair growth, runny nose, skin inflammation and itching, decreased heart rate.
Rare side effects(may affect up to 1 in 1,000 people)
Eye effects:
Thinning of the surface of the eye, inflammation of the glands of the eyelid, broken blood vessel in the eye, crusts on the eyelid, abnormal growth and positioning of the eyelashes.
Other effects:
Nervousness, irregular heart rate, hair loss, voice disorders, difficulty breathing, cough, throat irritation, hives, abnormal liver blood test values, skin discoloration, thirst, fatigue, unusual sensation inside the nose, colored urine, pain in hands and feet.
Frequency not known(cannot be estimated from the available data)
Eye effects:
Drooping eyelid (causing the eye to be half-closed), sunken eyes (the eyes appear more recessed), changes in iris color (the colored part of the eye).
Other effects:
Skin rash, heart failure, chest pain, stroke, fainting, depression, asthma, increased heart rate, tingling or numbness, palpitations, swelling of the legs, bad taste.
Additionally:
Travoprost/Timolol Stada is an association of 2 active substances, travoprost and timolol. Like other eye medicines, travoprost and timolol (a beta-blocker) are absorbed into the bloodstream. This may cause side effects similar to those seen with beta-blocker medicines given by mouth or injection. The incidence of side effects after administration in the eyes is lower than with medicines given by mouth or injection.
The following side effects have been observed with the class of beta-blockers used to treat eye conditions or with travoprost alone:
Eye effects:
Inflammation of the eyelid, inflammation of the cornea, detachment of the layer under the retina that contains blood vessels which can cause vision changes after filtration surgery, decreased corneal sensitivity, corneal erosion (damage to the outer layer of the eyeball), double vision, eye discharge, swelling around the eye, itching of the eyelid, abnormal turning out of the lower eyelid with redness, irritation and increased tear production, blurred vision (sign of cataract), swelling of a part of the eye (uvea), eczema of the eyelids, halo vision, decreased sensitivity in the eye, pigmentation inside the eye, dilated pupils, change in eyelash color, change in eyelash texture.
Other effects:
- Ear and labyrinth disorders: dizziness with a feeling of movement, ringing in the ears.
- Heart and circulation: slow heart rate, palpitations, edema (fluid accumulation), changes in heart rhythm or rate, congestive heart failure (heart disease with difficulty breathing and swelling of the feet and legs due to fluid accumulation), type of heart rhythm disorder, heart attack, decreased blood pressure, Raynaud's phenomenon, cold hands and feet, reduced blood flow to the brain.
- Respiratory: constriction of the airways in the lungs (mainly in patients with pre-existing disease), nasal congestion or stuffy nose, sneezing (due to allergy), difficulty breathing, nosebleeds, dry nose.
- Nervous system and general disorders: difficulty sleeping (insomnia), nightmares, memory loss, hallucinations, loss of strength and energy, anxiety (excessive emotional distress).
- Gastrointestinal: altered taste, nausea, indigestion, diarrhea, dry mouth, abdominal pain, vomiting and constipation.
- Allergic reactions: increased allergic symptoms, generalized allergic reactions including swelling under the skin that can occur in areas such as the face and limbs and can obstruct the airways, causing difficulty swallowing or breathing, hives, localized and generalized rash, itching, sudden and severe allergic reaction that can be life-threatening.
- Skin: rash with a white, scaly appearance (psoriasiform rash) or worsening of psoriasis, skin peeling, abnormal hair texture, skin inflammation with redness and itching, change in hair color, loss of eyelashes, itching, abnormal hair growth, redness of the skin.
- Muscle: increased signs and symptoms of myasthenia gravis (muscle disorder), unusual sensations such as tingling, muscle weakness/fatigue, muscle pain not caused by exercise, joint pain.
- Kidney and urinary disorders: difficulty and pain when urinating, involuntary urination.
- Reproduction: sexual dysfunction, decreased sexual desire.
- Metabolism: low blood sugar levels, increased prostate cancer marker.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Travoprost/Timolol Stada
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton, label, and bottle, after "EXP". The expiry date is the last day of the month indicated.
Before opening, this medicine does not require any special storage temperature.Keep the bottle in the outer carton to protect it from light.
After first opening, this medicine does not require any special storage conditions.
To avoid infections, you must discard the bottle 4 weeks after first openingand use a new bottle. Note the date of opening on the spaces provided on the label of each bottle and carton.
Medicines should not be disposed of via wastewater or household waste. Dispose of the packaging and any unused medicine in the pharmacy's SIGRE collection point. Ask your pharmacist how to dispose of the packaging and any unused medicine. This will help protect the environment.
6. Package Contents and Additional Information
Composition ofTravoprost/Timolol Stada
- The active substances are travoprost and timolol.
Each ml of solution contains 40 micrograms of travoprost and 5 mg of timolol (as timolol maleate).
- The other ingredients are benzalkonium chloride, macrogolglycerol hydroxystearate 40, trometamol, disodium edetate, boric acid (E284), mannitol (E421), sodium hydroxide (for pH adjustment), and water for injections or purified water.
Appearance and Package Contents of the Product
Travoprost/Timolol Stada is a clear, colorless, aqueous solution, practically free of particles, available in a 5 ml plastic bottle with a colorless nozzle and an opaque white cap with a tamper-evident seal.
Each bottle is enclosed in a carton. Each bottle contains 2.5 ml of solution.
The product is available in the following package sizes:
Packages containing 1, 3, or 6 bottles.
Not all package sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder
Laboratorio STADA, S.L.
Frederic Mompou, 5
08960 Sant Just Desvern (Barcelona)
Manufacturer
Pharmathen S.A.
6, Dervenakion str.,
Pallini, Attiki 15351
Greece
or
Balkanpharma-Razgrad AD
68 Aprilsko vastanie Blvd.
Razgrad, 7200
Bulgaria
or
STADA Arzneimittel AG
Stadastrasse 2 – 18
Bad Vilbel 61118
Germany
or
Centrafarm Services B.V.
Van de Reijstraat 31-E
4814 NE Breda
Netherlands
or
JADRAN - GALENSKI LABORATORIJ d.d.
Svilno 20,
Rijeka, 51000
Croatia
This medicine is authorized in the Member States of the European Economic Area under the following names:
Belgium Travoprost/Timolol EG 40 microgram/ml + 5mg/ml oogdruppels, oplossing
Luxembourg Travoprost/Timolol EG 40 microgrammes/ml + 5mg/ml collyre en solution
Estonia Travoprost/Timolol STADA 40 mikrogrammi/5 mg/ml silmatilgad, lahus
Latvia Travoprost/Timolol STADA 40 mikrogrami/ 5 mg/ml acu pilieni, škidums
Lithuania Travoprost/Timolol STADA 40 mikrogramu/ 5 mg/ml akiu lašai, tirpalas
Germany Travoprost/Timolol AL 40 Mikrogramm/ml + 5 mg/ml Augentropfen, Lösung
Denmark Travoprost/Timolol STADA 40 mikrogram/ml + 5 mg/ml øjendråber, opløsning
Spain Travoprost/Timolol STADA 40 microgramos/ml + 5 mg/ml colirio en solución
Finland Travoprost/Timolol STADA 40 mikrogram/ml + 5 mg/ml silmätipat, liuos
France TRAVOPROST/TIMOLOL EG 40 microgrammes/ml + 5 mg/ml, collyre en solution
Italy Travoprost e Timololo EG
Netherlands Travoprost/Timolol CF 0,04/5 mg/ml, oogdruppels, oplossing
Sweden Travoprost/Timolol STADA 40 mikrogram/ml + 5 mg/ml ögondroppar, lösning
Date of the last revision of this leaflet:June 2022
Detailed and up-to-date information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price7.09 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to TRAVOPROST/TIMOLOL STADA 40 micrograms/mL + 5 mg/mL Eye Drops SolutionDosage form: EYEDROP, 10 mg/ml + 5 mg/mlActive substance: timolol, combinationsManufacturer: Novartis Europharm LimitedPrescription requiredDosage form: EYEDROP, 0.3 mg/ml + 5 mg/mlActive substance: timolol, combinationsManufacturer: Brill Pharma S.L.Prescription requiredDosage form: EYE DROP, 0.3 mg/ml + 5 mg/mlActive substance: timolol, combinationsManufacturer: Laboratorio Stada S.L.Prescription required
Online doctors for TRAVOPROST/TIMOLOL STADA 40 micrograms/mL + 5 mg/mL Eye Drops Solution
Discuss questions about TRAVOPROST/TIMOLOL STADA 40 micrograms/mL + 5 mg/mL Eye Drops Solution, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions