
Toujeo 300 unidades/ml solostar solucion inyectable en pluma precargada

How to use Toujeo 300 unidades/ml solostar solucion inyectable en pluma precargada
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Toujeo 300 units/ml SoloStar solution for injection in a pre-filled pen
Insulin glargine
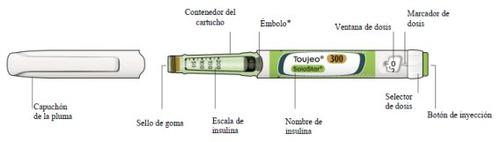
Each SoloStar pen delivers 1-80 units in steps of 1 unit.
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack and other information
- What is Toujeo and what is it used for
- What you need to know before you use Toujeo
- How to use Toujeo
- Possible side effects
- Storage of Toujeo
- Contents of the pack and other information
1. What is Toujeo and what is it used for
Toujeo contains insulin, called “insulin glargine”. This is a modified insulin that is very similar to human insulin.
Toujeo contains three times more insulin in 1 ml than standard insulin that contains 100 units/ml.
It is used to treat diabetes mellitus in adults, adolescents, and children from 6 years of age. Diabetes mellitus is a disease where your body does not produce enough insulin to control your blood sugar levels.
Toujeo lowers your blood sugar levels over a long period of time. It is used once a day. If needed, you can change the time of your injection because this medicine lowers your blood sugar levels over a long period of time (for more information, see section 3).
2. What you need to know before you use Toujeo
Do not use Toujeo
- if you are allergic to insulin glargine or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Consult your doctor, pharmacist, or nurse before starting Toujeo
Follow strictly the instructions on dosage, monitoring (blood and urine tests), diet, and physical activity (physical work and exercise) and injection technique established with your doctor.
You should pay special attention to the following:
- Low blood sugar levels (hypoglycaemia). If your blood sugar level is too low, follow the guidelines for hypoglycaemia (see information in the box at the end of this leaflet).
- If you change the type, brand, or manufacturer of insulin, you may need to change your insulin dose.
- Pioglitazone. See “Pioglitazone used with insulin”.
- Make sure to use the correct insulin. Medication errors have been reported due to confusion between insulins, particularly between long-acting and rapid-acting insulins. Always check the label of your insulin before each injection to avoid confusion between Toujeo and other insulins.
- Never use a syringe to withdraw Toujeo from your SoloStar pre-filled pen. This is to avoid dosing errors and possible overdoses that can lead to low blood sugar. See section 3.
- If you are blind or have poor eyesight, do not use the pre-filled pen without help, as you cannot read the dose window on the pen. Ask for help from another person with good eyesight and trained in the use of the pen. If you have poor eyesight, see section 3.
Changes in the skin at the injection site
The injection site should be rotated to avoid changes in the skin, such as lumps under the skin. Insulin may not work well if injected into a lumpy area (see How to use Toujeo). Contact your doctor if you are currently injecting into a lumpy area, before starting to inject into a different area. Your doctor may advise you to check your blood sugar levels more closely and adjust your insulin or the dose of your other anti-diabetic medications.
Illnesses and injuries
The management of your diabetes may require extra care in the following situations (e.g. blood and urine tests):
- If you are ill or have a major injury. Your blood sugar level may increase (hyperglycaemia).
- If you do not eat enough. Your blood sugar level may fall too low (hypoglycaemia).
In most cases, you will need to talk to a doctor. Contact your doctor as soon as you feel ill or have an injury.
If you have type 1 diabetes and are ill or have an injury:
- Do not stop taking your insulin.
- Keep taking enough carbohydrates.
Always inform the people in charge of your care or treatment that you have diabetes.
Treatment with insulin can cause your body to produce antibodies to insulin (substances that act against insulin). However, only in very rare cases will this require a change in your insulin dose.
Travel
Before travelling, consult your doctor. You may need to discuss:
- Whether your type of insulin is available in the country you are visiting.
- How to get insulin, needles, and other supplies.
- How to store your insulin correctly during travel.
- The timing of your meals and insulin administration.
- The possible effects of crossing time zones.
- Health risks in the countries you are visiting.
- What to do in emergency situations when you are not feeling well or become ill.
Children and adolescents
This medicine should not be given to children under 6 years of age because there is no experience with Toujeo in this age group.
Using Toujeo with other medicines
Tell your doctor, pharmacist, or nurse if you are taking, have recently taken, or might take any other medicines.
Some medicines may change your blood sugar level. This may mean that your insulin dose needs to be changed. Therefore, before taking any medicine, ask your doctor if it will affect your blood sugar level and what action to take if necessary. You also need to be careful when you stop taking a medicine.
Your blood sugar level may decrease (hypoglycaemia) if you take:
- Other medicines for diabetes.
- Disopyramide - for certain heart problems.
- Fluoxetine - for depression.
- Antibiotics of the sulphonamide group.
- Fibrates - to reduce high blood fat levels.
- Monoamine oxidase inhibitors (MAOIs) - for depression.
- Angiotensin-converting enzyme (ACE) inhibitors - for heart problems or high blood pressure.
- Medicines to relieve pain and lower fever, such as pentoxifylline, propoxyphene, and salicylates (such as acetylsalicylic acid).
- Pentamidine - for some parasite infections. This can cause low blood sugar levels that are sometimes followed by very high blood sugar levels.
Your blood sugar level may increase (hyperglycaemia) if you take:
- Corticosteroids such as cortisone - for inflammation.
- Danazol - for endometriosis.
- Diazoxide - for high blood pressure.
- Protease inhibitors - for HIV.
- Diuretics - for high blood pressure or fluid retention.
- Glucagon - for very low blood sugar levels.
- Isoniazid - for tuberculosis.
- Somatropin - a growth hormone.
- Thyroid hormones - for thyroid gland problems.
- Oestrogens and progestogens - such as the contraceptive pill for birth control.
- Clozapine, olanzapine, and phenothiazine derivatives - for mental health problems.
- Adrenergic agonists such as adrenaline (epinephrine), salbutamol, and terbutaline - for asthma.
Your blood sugar level may increase or decrease if you take:
- Beta-blockers or clonidine - for high blood pressure.
- Lithium salts - for mental health problems.
Beta-blockers
Beta-blockers, like other “sympatholytic medicines” (such as clonidine, guanethidine, reserpine - for high blood pressure), may make it more difficult to recognise the warning signs of low blood sugar (hypoglycaemia). They may even hide or interrupt the first signs of low blood sugar.
Pioglitazone used with insulin
Some patients with type 2 diabetes mellitus of long duration and previous heart disease or stroke who were treated with pioglitazone and insulin developed heart failure. Tell your doctor as soon as possible if you experience signs of heart failure such as unusual shortness of breath or rapid weight gain or localised swelling (oedema). Tell your doctor as soon as possible.
If any of the above applies to you (or if you are not sure), tell your doctor, pharmacist, or nurse before using Toujeo.
Using Toujeo with alcohol
Your blood sugar level may increase or decrease if you drink alcohol. You should check your blood sugar level more often.
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant, or are planning to have a baby, ask your doctor for advice before taking this medicine. Your insulin dose may need to be changed during pregnancy and after delivery. It is very important for the health of your baby and for you to keep your diabetes under control and to prevent hypoglycaemia.
If you are breast-feeding, ask your doctor for advice. You may need to adjust your insulin dose and diet.
Driving and using machines
Low or high blood sugar levels or problems with your eyesight may affect your ability to drive and use machines. Your concentration may be affected. This can be dangerous for you and others.
Ask your doctor whether you can drive if:
- Your blood sugar level is often too low.
- You have difficulty recognising when your blood sugar level is too low.
Toujeo contains sodium
This medicine contains less than 1 mmol (23 mg) of sodium per dose, i.e. it is essentially “sodium-free”.
3. How to use Toujeo
Follow exactly the instructions for administration of this medicine given by your doctor. If you are not sure, consult your doctor, pharmacist, or nurse again.
Although Toujeo contains the same active substance as insulin glargine 100 units/ml, these medicines are not interchangeable. Switching from one insulin treatment to another requires medical prescription, supervision, and monitoring of blood glucose. For more information, consult your doctor.
How much to use
The Toujeo SoloStar pre-filled pen can deliver doses of 1 to 80 units in one injection, in steps of 1 unit.
The dose window on the SoloStar pen shows the number of units of Toujeo to be injected. Do not adjust the dose.
Depending on your lifestyle, your blood sugar tests, and your previous insulin, your doctor will tell you:
- How much Toujeo you need each day and at what time.
- When to check your blood sugar level and if you need to do urine tests.
- When you may need higher or lower doses.
Toujeo is a long-acting insulin. Your doctor may tell you to use it with a short-acting insulin or with other medicines for high blood sugar levels.
If you use more than one insulin, always check that you are using the correct insulin by checking the label on the insulin before each injection. Medication errors have been reported due to confusion between insulins, particularly between long-acting and rapid-acting insulins. The dose “300” is highlighted in gold on the label of your Toujeo SoloStar pre-filled pen. Consult your doctor and pharmacist if you have any doubts.
Many factors can influence your blood sugar level. You should know these factors so that you can react correctly to changes in your blood sugar level and prevent it from becoming too high or too low. For more information, see the box at the end of this leaflet.
Flexibility in administration time
- Use Toujeo once a day, preferably at the same time each day.
- When needed, you can inject up to 3 hours before or after your usual time.
Use in elderly patients (65 years and older)
If you are 65 years or older, tell your doctor, as you may need a lower dose.
If you have kidney or liver problems
If you have kidney or liver problems, tell your doctor, as you may need a lower dose.
Before injecting Toujeo
- Read the instructions for use that appear in this leaflet.
- If you do not follow the instructions completely, you may get too much or too little insulin.
How to inject
- Toujeo is injected under the skin (subcutaneously or “SC”).
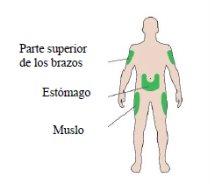
- Inject into the front of your thighs, the upper part of your arms, or the front of your waist (abdomen).
- Change the injection site each day within an injection area. This will reduce the risk of the skin shrinking or thickening (for more information, see “Other side effects” in section 4).
To prevent the possible transmission of diseases, insulin pens should never be used by more than one person, even if the needle is changed.
Always insert a new sterile needle before each injection. Never re-use needles. If you re-use a needle, you increase the risk of blocked needles and getting too much or too little insulin.
Dispose of the used needle in a puncture-resistant container or as instructed by your pharmacist or local authorities.
Do not use Toujeo
- In a vein, this will change the way it works and may cause your blood sugar level to fall too low.
- In an insulin pump.
- If particles appear in the insulin. The solution should be clear, colourless, and water-like.
Never use a syringe to withdraw Toujeo from your SoloStar pre-filled pen, as you may experience a severe overdose. See section 2.
If the SoloStar pen is damaged, it is because it has not been stored correctly. If you are not sure if it is working correctly or if your blood sugar control gets worse for no reason:
- Dispose of the pen and use a new one.
- If you think you have problems with your pen, tell your doctor, pharmacist, or nurse immediately.
If you use more Toujeo than you should
If you have injected too much medicine, your blood sugar level may fall too low. Check your blood sugar level and eat more to prevent your blood sugar level from falling too low. If your blood sugar level falls too low, see the box at the end of this leaflet.
If you forget to use Toujeo
When needed, Toujeo can be injected up to 3 hours before or after your usual time.
If you have missed a dose of Toujeo or if you have not injected enough insulin, your blood sugar level may increase too much (hyperglycaemia):
- Do not inject a double dose to make up for the missed dose.
- Check your blood sugar level and inject your next dose at the usual time.
- For more information on the treatment of hyperglycaemia, see the box at the end of this leaflet.
If you stop using Toujeo
Do not stop your treatment without consulting your doctor. If you do, this could lead to very high blood sugar levels and an increase in acid in the blood (ketoacidosis).
If you have any further questions on the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them.
If you notice signs that your blood sugar level is too low (hypoglycemia),act immediately to raise your blood sugar level (see the box at the end of this leaflet).
Hypoglycemia (low blood sugar) can be very serious and is very common during insulin treatment (it can affect more than 1 in 10 people).
Low blood sugar means that there is not enough sugar in the blood.
If your blood sugar level drops too low, you may faint (lose consciousness).
A severe low blood sugar level can cause brain damage and can be potentially fatal. For more information, see the box at the end of this leaflet.
Severe allergic reactions(rare, may affect up to 1 in 1,000 people). The signs may include rash and itching all over the body, swelling of the skin or mouth, difficulty breathing, feeling of dizziness (drop in blood pressure) with rapid heartbeat and sweating. Severe allergic reactions can be potentially fatal. Inform your doctor immediately if you notice the signs of a severe allergic reaction.
Other Adverse Effects
Tell your doctor, pharmacist, or nurse if you notice any of the following adverse effects:
- Changes in the skin at the injection site:
If insulin is injected too frequently in the same place, the fatty tissue can shrink (lipoatrophy, may affect up to 1 in 100 people) or become thicker (lipohypertrophy), (may affect up to 1 in 10 people). Lumps under the skin can also occur due to the accumulation of a protein called amyloid (cutaneous amyloidosis; the frequency of this is unknown). Insulin may not work very well if injected into a lumpy area. Change the injection site to help avoid these skin changes.
Frequent:may affect up to 1 in 10 people
- Skin reactions and allergic reactions at the injection site: the signs may include redness, intense pain when injecting, itching, hives, swelling, or inflammation. These reactions can spread around the injection site. Most minor insulin reactions usually disappear within a few days or weeks.
Rare:may affect up to 1 in 1,000 people
- Ocular reactions: a significant change in blood sugar control (improvement or worsening) can affect your vision. If you have a diabetes-related eye disorder called "proliferative retinopathy", episodes of very low blood sugar levels can cause temporary vision loss.
- Swelling of the calves and ankles caused by temporary water retention in the body.
Very Rare:may affect up to 1 in 10,000 people
- Change in taste (dysgeusia).
- Muscle pain (myalgia).
Tell your doctor, pharmacist, or nurse if you notice any of the above adverse effects.
Reporting Adverse Effects
If you experience any type of adverse effect, consult your doctor, pharmacist, or nurse, even if it is a possible adverse effect that is not listed in this leaflet. You can also report them directly through the national reporting system included in Appendix V. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of Toujeo
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiration date stated on the carton and on the label of the pen after CAD. The expiration date is the last day of the month indicated.
Before First Use
Store in a refrigerator (between 2°C and 8°C).
Do not freeze or place near the freezer or a cold accumulator.
Keep the pen in the outer packaging to protect it from light.
After First Use or if Carried as a Reserve
Do not store the pen in the refrigerator. The pen can be stored for a maximum of 6 weeks below 30°C, protected from direct heat or direct light. Discard the pen after this period. Do not leave your insulin in a car on an exceptionally hot or cold day. When not in use, always put the pen cap on to protect it from light.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of the packaging and medicines you no longer need. This will help protect the environment.
6. Container Contents and Additional Information
Composition of Toujeo
- The active substance is insulin glargine. Each ml of solution contains 300 units of insulin glargine (equivalent to 10.91 mg). Each pen contains 1.5 ml of injectable solution equivalent to 450 units.
The other components are: zinc chloride, metacresol, glycerol, water for injectable preparations, sodium hydroxide (see section 2 "Toujeo contains sodium") and hydrochloric acid (for pH adjustment).
Appearance of Toujeo and Container Contents
Toujeo is a clear and colorless solution.
Each pen contains 1.5 ml of injectable solution (equivalent to 450 units).
Packaging of 1, 3, 5, and 10 pre-filled pens.
Only some pack sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Sanofi-Aventis Deutschland GmbH, D-65926 Frankfurt am Main, Germany.
You can request more information about this medicine by contacting the local representative of the marketing authorization holder:
Belgium Sanofi Belgium Tel: +32 (0)2 710 54 00 | Lithuania UAB sanofi-aventis Lietuva Tel: +370 5 2755224 |
Bulgaria SANOFI BULGARIA EOOD Tel: +359 (0)2 970 53 00 | Luxembourg Sanofi Belgium Tel: +32 (0)2 710 54 00 (Belgium) |
Czech Republic sanofi-aventis, s.r.o. Tel: +420 233 086 111 | Hungary sanofi-aventis zrt., Hungary Tel: +36 1 505 0050 |
Denmark Sanofi A/S Tel: +45 45 16 70 00 | Malta Sanofi S.p.A Tel: 39 02 39394275 |
Germany Sanofi-Aventis Deutschland GmbH Tel: 0800 52 52 010 Tel from abroad: +49 69 305 21 131 | Netherlands sanofi-aventis Netherlands B.V. Tel: +31 20 245 4000 |
Estonia sanofi-aventis Estonia OÜ Tel: +372 627 34 88 | Norway sanofi-aventis Norge AS Tel: +47 67 10 71 00 |
Greece sanofi-aventis AEBE Tel: +30 210 900 16 00 | Austria sanofi-aventis GmbH Tel: +43 1 80 185 – 0 |
Spain sanofi-aventis, S.A. Tel: +34 93 485 94 00 | Poland sanofi-aventis Sp. z o.o. Tel: +48 22 280 00 00 |
France sanofi-aventis France Tel: 0 800 222 555 Call from abroad: +33 1 57 63 23 23 | Portugal Sanofi - Produtos Farmacêuticos, Lda Tel: +351 21 35 89 400 |
Croatia sanofi-aventis Croatia d.o.o. Tel: +385 1 600 34 00 | Romania Sanofi Romania SRL Tel: +40 (0) 21 317 31 36 |
Ireland sanofi-aventis Ireland Ltd. T/A SANOFI Tel: +353 (0) 1 403 56 00 | Slovenia sanofi-aventis d.o.o. Tel: +386 1 560 48 00 |
Iceland Vistor hf. Tel: +354 535 7000 | Slovakia sanofi-aventis Pharma Slovakia s.r.o. Tel: +421 2 33 100 100 |
Italy Sanofi S.p.A. Tel: 800 131212 (technical questions) 800 536389 (other questions) | Finland Sanofi Oy Tel: +358 (0) 201 200 300 |
Cyprus sanofi-aventis Cyprus Ltd. Tel: +357 22 871600 | Sweden Sanofi AB Tel: +46 (0)8 634 50 00 |
Latvia sanofi-aventis Latvia SIA Tel: +371 67 33 24 51 | United Kingdom Sanofi Tel: +44 (0) 845 372 7101 |
Date of Last Revision of this Leaflet:
Detailed information on this medicine is available on the European Medicines Agency website: http://www.ema.europa.eu.
HYPGLYCEMIA AND HYPERGLYCEMIA If you are taking insulin, you should always carry the following:
Hyperglycemia (high blood sugar levels) If your blood sugar level is too high (hyperglycemia), you may not have injected enough insulin. Reasons why hyperglycemia may occur Some examples are:
Warning signs of hyperglycemia Thirst, increased need to urinate, fatigue, dry skin, redness of the face, loss of appetite, low blood pressure, rapid heartbeat, and the presence of glucose and ketone bodies in the urine. Stomach pain, deep and rapid breathing, feeling sleepy or passing out (loss of consciousness) may be signs of a serious condition (ketoacidosis) caused by lack of insulin. What to do if you experience hyperglycemia?
Hypoglycemia (low blood sugar levels) If your blood sugar level drops too low, you may pass out (lose consciousness). Severe hypoglycemia can cause a heart attack or brain damage and can be life-threatening. You should learn to recognize the signs that indicate your blood sugar level is dropping, so you can take the necessary measures to prevent the situation from getting worse. Reasons why hypoglycemia may occur Some examples are:
It is also more likely to occur if:
Warning signs of hypoglycemia The first signs can be generally in your body. Examples of signs that your blood sugar level is dropping too low or too quickly are: sweating, moist and sticky skin, anxiety, rapid and irregular heartbeat, high blood pressure, and palpitations. These signs often occur before signs of low blood sugar in the brain appear. The signs in your brain include: headache, feeling very hungry, nausea, vomiting, feeling tired, drowsiness, agitation, sleep problems, aggressive behavior, difficulty concentrating, reduced reaction capacity, depression, feeling confused, difficulty speaking (sometimes, complete loss of speech), change in vision, tremors, inability to move (paralysis), tingling in hands or arms, feeling numb and tingling often around the mouth, feeling dizzy, loss of self-control, inability to take care of oneself, seizures, loss of consciousness. Situations in which the warning signs of hypoglycemia may be less clear: The first warning signs of hypoglycemia may change, weaken, or be absent if:
In these cases, you may experience severe hypoglycemia (and even pass out) before you realize what is happening. Always be familiar with your warning signs. If necessary, you may need to perform blood sugar tests more frequently. This can help identify mild hypoglycemic episodes. If you have difficulty recognizing your warning signs, you should avoid situations (such as driving a car) that could put you or others at risk due to hypoglycemia. What to do if you experience hypoglycemia?
Ask your doctor or nurse if you are not sure what to eat. With Toujeo, recovery from low blood sugar may be delayed because it has a prolonged action.
What should others do if you have hypoglycemia? Inform your family, friends, and people close to you that you need urgent medical help if you are unable to swallow or if you pass out (lose consciousness). You will need an injection of glucose or glucagon (a medication that increases blood sugar levels). These injections are justified even if you are not sure if you have hypoglycemia. It is recommended to check your blood sugar level immediately after ingesting glucose to confirm that you actually have hypoglycemia. |
Toujeo 300 units/ml solution for injection in pre-filled pen (SoloStar)
INSTRUCTIONS FOR USE
Read this first
Toujeo SoloStar contains 300 units/ml of insulin glarginein a 1.5 ml pre-filled disposable pen
- Never reuse needles. If you do, you may not receive the necessary dose (underdosing) or receive too much (overdosing), as the needle may become blocked.
- Never use a syringe to extract insulin from your pen. If you do, you will extract too much insulin. The graduation on most syringes is intended only for non-concentrated insulin.
Important information
Never share your pen - it is only for you.
Never use your pen if it is damaged or if you are not sure it is working correctly.
Always perform a safety test.
Always carry a spare pen and needles in case they are lost or stop working.
Learning to inject
- Ask your doctor, pharmacist, or nurse how to inject before using your pen.
- Ask for help if you have problems handling your pen, for example if you have vision problems.
- Read all these instructions before using your pen. If you do not follow all these instructions, you may receive too much insulin or too little.
Do you need help?
If you have questions about your pen or your diabetes, ask your doctor, pharmacist, or nurse or call the sanofi-aventis number listed at the beginning of this leaflet.
Additional items you will need:
- a new sterile needle (see STEP 2).
- a puncture-resistant container for used needles and pens.
Injection sites
Get to know your pen
- You will not see the plunger until you have injected a few doses.
STEP 1: Check your pen
Remove a new pen from the refrigerator at least 1 hour before your injection. Injecting cold insulin is more painful.
ACheck the name and expiration date on the label of your pen.
- Make sure you have the correct insulin. This is especially important if you have other pens.
- Do not use the pen after the expiration date.

BRemove the pen cap.
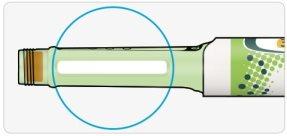
CCheck that the insulin is clear.
- Do not use the pen if the insulin is cloudy, has color, or contains particles.
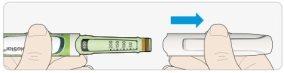
STEP 2: Attach a new needle
Always use a new sterile needle for each injection. This will help prevent needle blockage, contamination, and infection.
Only use needles compatible with Toujeo (e.g., BD, Ypsomed Artsana, or Owen Mumford needles).
ATake a new needle and remove the protective seal.
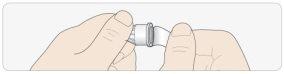
BKeep the needle straight and screw it onto the pen until it is fixed. Do not overtighten.
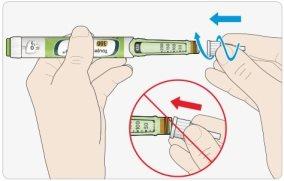
CRemove the outer needle cap. Save it for later.
DRemove the inner needle cap and discard it.
Handling needles
- Be careful when handling needles to avoid puncture injuries and cross-infection.
STEP 3: Perform a safety test
Always perform a safety test before each injection to:
- check that your pen and needle are working correctly.
- make sure you receive the correct dose of insulin.
ASelect 3 units by turning the dose selector until the dose marker is between the 2 and 4 marks.
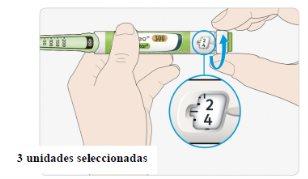
BPress the injection button all the way down.
- If insulin comes out of the needle tip, your pen is working correctly.
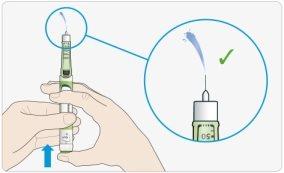
If no insulin comes out:
- You may need to repeat this step up to 3 times before you see insulin come out.
- If no insulin comes out after the third time, the needle may be blocked. If this happens:
- change the needle (see STEP 6 and STEP 2),
- then repeat the safety test (STEP 3).
- Do not use your pen if insulin still does not come out of the needle tip. Use a new pen.
- Do not use a syringe to extract insulin from your pen.
If you see air bubbles
- You may see air bubbles. This is normal; they will not harm you.
STEP 4: Select the dose
Never select the dose or press the injection button while the needle is not attached, as you may damage your pen.
AMake sure the needle is attached and the dose is set to "0".

BTurn the dose selector until the dose marker is aligned with your dose.
- If you exceed your dose, you can turn it back.
- If there are not enough units left in your pen to administer your dose, the dose selector will stop at the number of units left.
- If you cannot select your full prescribed dose, divide the dose into two injections or use a new pen.
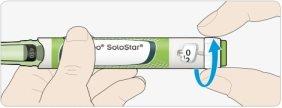
How to read the dose window
Even numbers are indicated on the same line as the dose marker:

30 units selected
Odd numbers are indicated as a line between the even numbers:

29 units selected
Insulin units in your pen
- Your pen contains a total of 450 units of insulin. You can select doses from 1 to 80 units in single-unit steps. Each pen contains more than one dose.
- You can see more or less how many units are left by looking at where the plunger is on the insulin scale.
STEP 5: Inject the dose
If you have trouble pressing the injection button, do not force it, as you may break your pen. See the next section for help.
AChoose an injection site as shown in the drawing.
BPush the needle into your skin, just as your doctor, pharmacist, or nurse has taught you.
- Do not press the injection button yet.
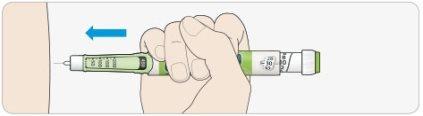
CPlace your thumb on the injection button. Press it all the way down and hold it down.
- Do not press the button at an angle: your thumb may block the dose selector and prevent it from turning.
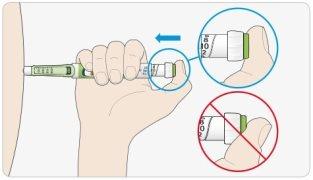
DHold the injection button down and when you see "0" in the dose window, count slowly to 5.
- This will ensure you receive your full dose.
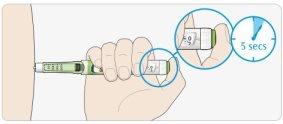
EAfter holding it down and counting slowly to 5, release the injection button. Then, remove the needle from your skin.
If you have trouble pressing the button:
- Change the needle (see STEP 6 and STEP 2) and then perform a safety test (see STEP 3).
- If you still have trouble pressing the button, use a new pen.
- Do not use a syringe to withdraw insulin from your pen.
STEP 6: Remove the needle
Be careful when handling needles to avoid puncture injuries and cross-infection.
Do not put the inner needle cap back on.
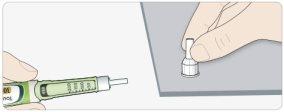
APut the outer needle cap on, and use it to unscrew the needle from the pen.
- To reduce the risk of accidental needlesticks, never put the inner cap back on.
- If someone else is giving you the injection or if you are giving the injection to someone else, be especially careful when removing or disposing of the needle.
- Follow the safety measures for removing and disposing of needles (contact your doctor, pharmacist, or nurse) to reduce the risk of accidental needlesticks and the transmission of infectious diseases.
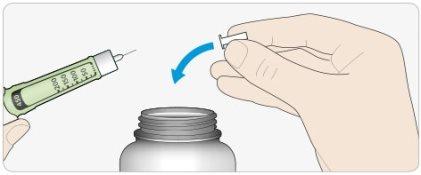
BDispose of the used needle in a puncture-resistant container, or as directed by your pharmacist or local authorities.
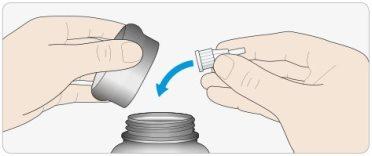
CPut the pen cap back on.
- Do not put the pen back in the refrigerator.
Use
- Use your pen for a maximum of 6 weeks after its first use.
How to store your pen
Before first use
- Store new pens in the refrigerator at a temperature between 2°C and 8°C.
- Do not freeze.
After first use
- Store your pen at room temperature, below 30 °C.
- Do not put your pen back in the refrigerator.
- Do not store your pen with the needle attached.
- Store your pen with the cap on.
How to care for your pen
Handle your pen with care
- Do not drop your pen or hit it against hard surfaces.
- If you think your pen may be damaged, do not try to repair it; use a new one.
Protect your pen from dust and dirt
- You can clean the outside of your pen with a damp cloth. Do not get your pen wet, wash it, or lubricate it, as this may damage it.
Disposing of your pen
- Remove the needle before disposing of your pen.
- Dispose of your used pen as directed by your healthcare professional or local authorities.
- Country of registration
- Average pharmacy price50.63 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Toujeo 300 unidades/ml solostar solucion inyectable en pluma precargadaDosage form: INJECTABLE, 100 U/mlActive substance: insulin glargineManufacturer: Eli Lilly Nederland B.V.Prescription requiredDosage form: INJECTABLE, UnknownActive substance: insulin glargineManufacturer: Sanofi-Aventis Deutschland GmbhPrescription requiredDosage form: INJECTABLE, UnknownActive substance: insulin glargineManufacturer: Sanofi-Aventis Deutschland GmbhPrescription required
Online doctors for Toujeo 300 unidades/ml solostar solucion inyectable en pluma precargada
Discuss questions about Toujeo 300 unidades/ml solostar solucion inyectable en pluma precargada, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















