TOBRAMICIN ALTAN 300 MG/5 ML SOLUTION FOR NEBULIZER INHALATION


How to use TOBRAMICIN ALTAN 300 MG/5 ML SOLUTION FOR NEBULIZER INHALATION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Tobramycin Altan300 mg/5 ml Solution for Inhalation by Nebulizer
Tobramycin
Read this package leaflet carefully before you start taking this medicine, because it contains important information for you.
- Keep this package leaflet, you may need to read it again.
If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this package leaflet. See section 4.
Contents of the Package Leaflet
- What is Tobramycin Altan and what is it used for
- What you need to know before you start using Tobramycin Altan
- How to use Tobramycin Altan
- Possible side effects
- Storage of Tobramycin Altan
- Contents of the pack and other information
1. What is Tobramycin Altan and what is it used for
Tobramycin Altan contains a medicine called tobramycin, which is an aminoglycoside antibiotic.
Antibiotics are used to treat bacterial infections and do not work for viral infections, such as the flu or the common cold. It is important that you follow the instructions regarding the dose, administration interval, and treatment duration indicated by your doctor. Do not store or reuse this medicine. If you have any leftover antibiotic after finishing the treatment, return it to the pharmacy for proper disposal. Do not throw away medicines via wastewater or household waste. |
What Tobramycin Altan is used for
Tobramycin Altan is used in patients aged 6 years and older who have cystic fibrosis, for the treatment of lung infections caused by a bacterium called Pseudomonas aeruginosa.
Tobramycin Altan combats the infection caused by the Pseudomonas bacterium in your lungs and helps improve your breathing.
How Tobramycin Altan works
When you inhale Tobramycin Altan, the antibiotic can reach your lungs directly to fight the bacteria that cause the infection. For better results with this medicine, follow the instructions in this package leaflet.
What is Pseudomonas aeruginosa?
It is a very common bacterium that infects almost all patients with cystic fibrosis at some point in their lives. Some of them do not get this infection until very late in life, while others get it very young.
This is one of the most damaging bacteria for people with cystic fibrosis. If the infection is not controlled properly, it can continue to damage your lungs, causing additional breathing problems.
Tobramycin Altan kills the bacteria that cause lung infections. This infection can be successfully controlled if the problem is addressed in an early stage.
2. What you need to know before you start using Tobramycin Altan
Do not useTobramycin
- if you are allergic to tobramycin, to any aminoglycoside antibiotic, or to any of the other ingredients of this medicine (listed in section 6).
If any of the above cases apply to you, do not take this medicine and consult your doctor.
Consult your doctor if you think you may be allergic.
Warnings and precautions:
Consult your doctor before starting to use Tobramycin if you suffer or have ever suffered from any of the following conditions:
- Hearing problems (including ringing in the ears and dizziness).
- Kidney problems
- Unusual difficulty breathing with wheezing or coughing, chest tightness
- Blood in your sputum (the matter you cough up)
- Muscle weakness that lasts or worsens over time, symptoms mostly related to myasthenia or Parkinson's disease.
If any of these cases apply to you, inform your doctor before using Tobramycin.
If you or members of your maternal family have a disease caused by a mitochondrial mutation (a genetic disease) or hearing loss due to antibiotics, it is recommended that you inform your doctor or pharmacist before taking this medicine. Certain mitochondrial mutations can increase your risk of hearing loss with this product. Your doctor may recommend genetic tests before administering tobramycin.
Inhaling medicines can cause chest tightness and wheezing, and this can occur with Tobramycin. Your doctor will supervise your first dose of Tobramycin and check your lung function before and after the dose. If you are not doing so, your doctor may have you use a bronchodilator (e.g., salbutamol) before using Tobramycin.
If you are using Tobramycin, Pseudomonas strains may become resistant to treatment over time. This means that over time, the medicine may not work as well as it should. Consult your doctor if you are concerned about this issue.
If administration is by injection, tobramycin can occasionally cause hair loss, dizziness, and kidney damage and may harm the fetus.
Children and adolescents
Tobramycin can be administered to children and adolescents from 6 years of age. Tobramycin cannot be administered to children under 6 years of age.
Elderly
If you are 65 years of age or older, your doctor may perform additional tests to decide if Tobramycin is a suitable treatment for you.
Using Tobramycin with other medicines
Tell your doctor or pharmacist if you are using or have recently used other medicines, including those obtained without a prescription.
Do not take the following medicines while using Tobramycin:
- Furosemide or ethacrynic acid, diuretics
- Intravenous urea or mannitol
- Other medicines that can damage your nervous system, kidneys, or ears.
The following medicines may increase the risk of adverse effects if administered while you are receiving injectionsof tobramycin:
- Amphotericin B, cephalothin, cyclosporine, tacrolimus, polymyxins: these medicines can damage your kidneys.
- Platinum compounds (such as carboplatin and cisplatin): these medicines can damage your kidneys or ears.
- Anticholinesterases (such as neostigmine and pyridostigmine) or botulinum toxin: these medicines can cause or worsen muscle weakness.
If you are taking one or more of the above medicines, discuss this with your doctor before using Tobramycin.
Do not mix or dilute Tobramycin with any other medicine in your nebulizer.
If you are taking several different treatments for cystic fibrosis, you should take them in the following order:
- Bronchodilator treatment, such as salbutamol
- Chest physiotherapy
- Other inhaled medicines
- Tobramycin, last.
Also, check this order with your doctor.
Pregnancy and breastfeeding
If you are pregnant or want to become pregnant, consult your doctor about the possibility that this medicine may harm you or the fetus.
It is not known if inhaling this medicine during pregnancy causes adverse effects.
When administered by injection, tobramycin and other aminoglycoside antibiotics can cause harm to the fetus, such as deafness.
If you are breastfeeding, consult your doctor before using any medicine.
Driving and using machines
Tobramycin should not affect your ability to drive and use machines.
3. How to use Tobramycin Altan
Follow the instructions for administering this medicine exactly as indicated by your doctor. In case of doubt, consult your doctor again.
How much of this medicine to use and how often to use it
- The recommended dose is the same for all people aged 6 years and older.
- Use twoampoules each day, for 28 days. Inhale the complete contents of one ampoule in the morning and another in the evening. Ideally, there should be an interval of 12 hours between doses.
- You must leave a minimum period of 6 hoursbetween the two Tobramycin inhalations.
- After taking your medicine for 28 days, you will then have a 28-day interval during which you should not inhale any dose of Tobramycin, before starting another cycle.
- It is important that you maintain the use of the product twice a day during your 28-day treatment period and that you maintain the 28-day treatment cycles, 28-day rest periods.
WITH Tobramycin Altan | WITHOUT Tobramycin Altan |
Take Tobramycin Altan twice a day, every day for 28 days | Do not take Tobramycin Altan during the next 28 days |
Repeat the cycle
If you use more Tobramycin than you should
If you inhale too much Tobramycin, your voice may become very hoarse. Make sure to inform your doctor as soon as possible. If you swallow Tobramycin, inform your doctor as soon as possible.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone: 91 562 04 20, indicating the medicine and the amount ingested.
If you forget to use Tobramycin
If you forget to use Tobramycin and it is at least 6 hours before your next dose, take a new dose as soon as possible. Otherwise, wait for your next dose. Do not take a double dose to make up for the forgotten doses.
Instructions for using Tobramycin
This part of the package leaflet explains how to use, care for, and handle Tobramycin. Read and follow these instructions carefully.
If you have any additional questions about using this medicine, consult your doctor or pharmacist.
The equipment you need to inhale Tobramycin
Tobramycin must be used with a reusable, clean, and dry nebulizer.
The LC PLUS nebulizer (manufactured by PARI GmbH) is suitable for use with Tobramycin.
Your doctor or physiotherapist may advise you on the correct use of Tobramycin and the equipment you need. You may need different nebulizers for your other inhaled medicines for cystic fibrosis.
Preparing Tobramycin for inhalation
- Wash your hands carefully with water and soap.
- Each aluminum bag of Tobramycin contains a tray with 14 ampoules. Cut or tear the bag. Remove one Tobramycin ampoule from the tray by gently pulling the lower tab to separate it from the rest of the ampoules. Put the tray back in the aluminum bag and store it in the refrigerator.
- Lay out the parts of your nebulizer on a clean and dry paper or cloth towel.
- Make sure you have the correct compressor and tube to connect the nebulizer and compressor.
- Carefully follow the instructions for using your nebulizer model, read the instruction manual provided by the manufacturer. Check that your nebulizer and compressor are working properly according to the manufacturer's instructions before starting to use your medicine.
Using Tobramycin with LC PLUS (PARI GmbH)
For more detailed instructions on using and caring for the nebulizer, read the instruction manual provided with the PARI LC PLUS.
- Remove the top part of the nebulizer by turning it counterclockwise and lifting it. Place the top part on the towel and put the nebulizer cup upright on the towel.
- Connect one end of the tube to the air outlet of the compressor. Make sure the tube fits perfectly. Connect the compressor to the power outlet.
- Open the Tobramycin ampoule by holding the lower tab with one hand and separating the top tip with the other hand, twisting it. Empty the entire contents of the ampoule into the nebulizer cup.
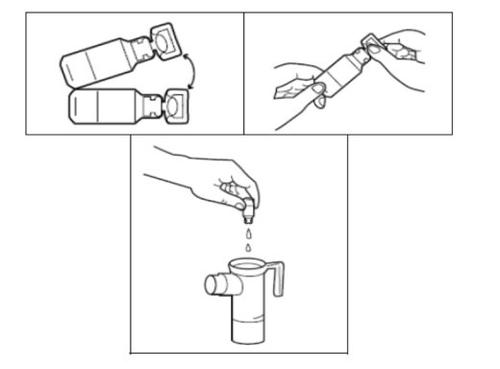
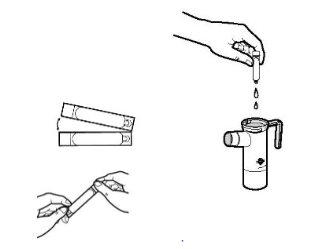
- Put the top part of the nebulizer back, fix the mouthpiece, and place the valve cap on the nebulizer. Then connect the compressor as indicated in the instruction manual of your PARI LC PLUS nebulizer.
- Turn on the compressor. Check that there is a constant mist coming out of the mouthpiece. If there is no mist, check all the tube connections and that the compressor is working properly.
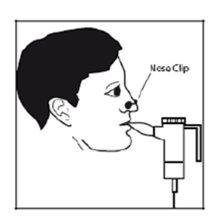
- Sit or stand in a way that allows you to breathe normally.
- Place the mouthpiece between your teeth and over the tip of your tongue. Breathe normally, but only through your mouth (you may find it helpful to use a nose clip). Try not to block the airflow with your tongue.
- Continue until all the Tobramycin Altan has been consumed and no more mist is produced. The complete inhalation should take around 15 minutes. You may hear a gurgling sound when the nebulizer cup is empty.
- Remember to clean and disinfect your nebulizer after treatment according to the manufacturer's instructions. Never use a dirty or clogged nebulizer. Do not share your nebulizer with other people.
If you are interrupted or need to cough or rest during administration, turn off the compressor to avoid wasting the medicine.
Turn the compressor back on when you are ready to restart the treatment. Omit this dose if your next dose is due in less than 6 hours.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Some side effects can be serious
If you experience any of the following side effects, stop using Tobramycin and inform your doctor immediately:
- Unusual difficulty breathing with wheezing or coughing and chest tightness
- Allergic reactions including hives and itching
If you experience any of the following side effects, inform your doctor immediately:
- Hearing loss (ringing in the ears is a sign of potential hearing loss), noises (such as whistling) in the ears
Your underlying lung disease may worsen while you are using Tobramycin. This may be due to lack of efficacy. Inform your doctor immediately if this occurs.
Some side effects are very common
These side effects may affect more than 1 in 10 people.
- Runny or stuffy nose, sneezing
- Voice alteration (hoarseness)
- Discoloration of the sputum
- Worsening of lung function test results
If any of these affect you severely, inform your doctor.
Some side effects are common
These side effects may affect up to 1 in 10 people.
- Feeling unwell
- Muscle pain
- Voice alteration with sore throat and difficulty swallowing (laryngitis)
If any of these affect you severely, inform your doctor.
Other side effects:
- Itching
- Itchy skin rash
- Skin rash
- Loss of voice
- Altered sense of taste
- Sore throat
If any of these affect you severely, inform your doctor.
If you have received Tobramycin at the same time or after repeated cycles of tobramycin or other aminoglycoside antibiotics injected, hearing loss has been reported as a side effect.
Injections of tobramycin or other aminoglycosides can cause allergic reactions, hearing problems, and kidney problems.
Patients with cystic fibrosis have various symptoms of the disease. These may even occur while taking Tobramycin but should not be more frequent or seem worse than before treatment.
Reporting of side effects
If you experience anyside effects, talk to your doctor or pharmacist, even if it is possible side effects not listed in this package leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines (www.notificaRAM.es). By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Tobramicina Altan
- Keep this medication out of sight and reach of children.
- Do not use this medication after the expiration date shown on the packaging, bag, or printed on the ampoule.
- Do not use this medication if you notice it becomes cloudy or if there are particles in the solution.
- Store in refrigerator(between 2°C and 8°C). If you do not have a refrigerator (e.g., during transportation of the medication), you can keep the aluminum bags (open or closed) at room temperature (not above 25°C) for a maximum of 28 days.
- Do not use the Tobramicina ampoules if you have stored them at room temperature for more than 28 days.
- Keep the ampoule in its original packaging to protect it from light. This medication usually has a light yellow color, which may vary and sometimes may be dark yellow. This does not affect the activity of this medication, as long as the storage instructions are followed.
You should never store an opened ampoule. Once an ampoule is opened, it must be used immediately, and any remaining product must be discarded.
6. Package Contents and Additional Information
Composition of Tobramicina Altan
- The active ingredient is tobramycin. One ampoule contains 300 mg of tobramycin as a single dose.
- The other components are sodium chloride, water for injectable preparations, sodium hydroxide, and sulfuric acid (to adjust the acidity level).
Appearance of the Product and Package ContentsTobramicina Altan is a clear, slightly yellow solution presented in a ready-to-use ampoule.
The ampoules are packaged in aluminum bags, one aluminum bag contains 7 ampoules, which correspond to 7 days of treatment.
Tobramicina Altan is available in packages of 56 ampoules, which are sufficient for one treatment cycle.
Not all package sizes may be marketed.
Marketing Authorization Holder:
Altan Pharmaceuticals, S.A.
C/ Cólquide, Nº 6, Portal 2, 1ª Planta, Oficina F.
Edificio Prisma,
Las Rozas, 28230 Madrid
Manufacturer:
Altan Pharmaceuticals, S.A.
Polígono Industrial de Bernedo, s/n
01118 Bernedo (Álava)
Spain
Altan Pharmaceuticals, S.A.
Avda. de la Constitución, 198-199
Polígono Industrial Monte Boyal, Casarrubios del Monte, 45950 Toledo
Spain
This medication is authorized in the following EU member states with the following names:
France: Tobramycine Zentiva 300 mg/5 ml Solution pour inhalation par nébuliseur
Germany: Tobramycin Zentiva 300 mg/ 5 ml Lösung für einen Vernebler
Italy: Tobramicina Altan 300 mg/5 ml soluzione per nebulizzatore
Portugal: Tobramicina Altan. 300 mg/ 5 ml Solução para Inalação por Nebulização
Spain: Tobramicina Altan. 300 mg/ 5 ml Solución para inhalación por nebulizador
United Kingdom: Tobramycin 300 mg/ 5 ml Nebuliser solution
Date of the last revision of this prospectus: October 2023
Other Sources of Information
Detailed and updated information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to TOBRAMICIN ALTAN 300 MG/5 ML SOLUTION FOR NEBULIZER INHALATIONDosage form: PULMONARY INHALATION, 300 mg / 4 mlActive substance: tobramycinManufacturer: Chiesi España S.A.U.Prescription requiredDosage form: PULMONARY INHALATION, 28 mg tobramycinActive substance: tobramycinManufacturer: Viatris Healthcare LimitedPrescription requiredDosage form: PULMONARY INHALATION, 300 mg/5 mlActive substance: tobramycinManufacturer: Accord Healthcare S.L.U.Prescription required
Online doctors for TOBRAMICIN ALTAN 300 MG/5 ML SOLUTION FOR NEBULIZER INHALATION
Discuss questions about TOBRAMICIN ALTAN 300 MG/5 ML SOLUTION FOR NEBULIZER INHALATION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions